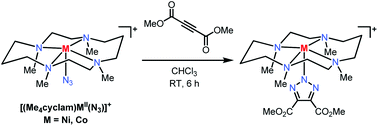
The studies described herein focus on the 1,3-dipolar cycloaddition reaction between first-row transition metal–azide complexes and alkyne reagents, i.e. an inorganic variant of the extensively used “click reaction”. The reaction between the azide complexes of biologically-relevant metals (e.g., Fe, Co and Ni) found in metalloenzyme active sites and alkyne reagents has been investigated as a proof-of-principle for a novel method of developing metalloenzyme triazole-based inhibitors. Six Fe, Co and Ni mono-azide complexes employing salen- and cyclam-type ligands have been synthesized and characterized. The scope of the targeted inorganic azide–alkyne click reaction was investigated using the electron-deficient alkyne dimethyl acetylenedicarboxylate. Of the six metal–azide complexes tested, the Co and Ni complexes of the 1,4,8,11-tetrametyl-1,4,8,11-tetraazacyclotetradecane (Me4cyclam) ligand showed a successful cycloaddition reaction and formation of the corresponding metal–triazolate products, which were crystallographically characterized. Moreover, use of less electron deficient alkynes resulted in a loss of cycloaddition reactivity. Analysis of the structural parameters of the investigated metal–azide complexes suggests that a more symmetric structure and charge distribution within the azide moiety is needed for the formation of a metal–triazolate product. Overall, these results suggest that a successful cycloaddition reaction between a metal–azide complex and an alkyne substrate is dependent both on the ligand and metal oxidation state, that determine the electronic properties of the bound azide, as well as the electron deficient nature of the alkyne employed.

 Please wait while we load your content...
Please wait while we load your content...