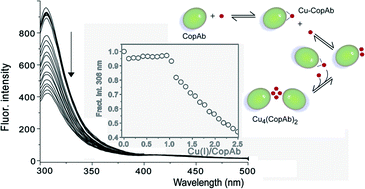
The Cu(I)-detoxifying P-type ATPase CopA from Bacillus subtilis contains two N-terminal soluble domains, CopAa and CopAb, connected by a short linker. This arrangement is extremely common in prokaryotic Cu(I) transporters and is also found amongst the multiple soluble domains of eukaryotic homologues. Previous studies of a protein containing only these domains (CopAab) revealed complex Cu(I)-binding properties: both domains are able to bind Cu(I) extremely tightly and, at levels of Cu(I) > 1 per CopAab, the protein undergoes dimerisation, yielding a highly luminescent multi-Cu(I) bound species (Singleton and Le Brun, Dalton Trans., 2009, 688–696). To investigate this complex Cu(I)-binding behaviour and, in particular, to determine the contributions of the two domains to the overall behaviour of the N-terminal part, we generated and purified each domain in isolation. Here, we report studies of the second domain, CopAb. The protein was found to bind Cu(I) with an extremely high affinity (K = ∼1 × 1018 M−1) and remained as a monomer up to a level of 1 Cu(I) per protein. Above this level, the protein dimerised, generating a weakly luminescent species. Studies of the acid–base properties of the binding motif Cys residues revealed pKa values of < ∼5 and ∼6.3, adding further support to the proposal that high Cu(I)-affinity is correlated with low proton affinity. Exchange of Cu(I) between the protein and a high affinity chelator was found to occur rapidly via Cu(I)-mediated association, a process that is relevant to in vivo Cu(I) trafficking. Overall, the Cu(I)-binding properties of CopAb are very similar to those of the two-domain protein CopAab, indicating that this domain plays a dominant role in determining the binding properties of CopAab.

 Please wait while we load your content...
Please wait while we load your content...