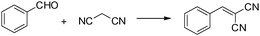mpg-C3N4 as a solid base catalyst for Knoevenagel condensations and transesterification reactions†
Fangzheng
Su
*a,
Markus
Antonietti
a and
Xinchen
Wang
*b
aDepartment of Colloid Chemistry, Max Planck Institute of Colloids and Interfaces, Research Campus Golm, D-14476 Golm, Potsdam, Germany. E-mail: fangzheng.su@mpikg.mpg.de; Fax: +49-331-5679502
bResearch Institute of Photocatalysis, State Key Laboratory Breeding Base of Photocatalysis, Fuzhou University, Fuzhou 350002, P. R. China. E-mail: xcwang@fzu.edu.cn
First published on 15th February 2012
Abstract
Deprotonated mesoporous graphitic carbon nitride (mpg-C3N4) was achieved by a simple method of treatment of as-prepared materials with different basic aqueous solutions (K2CO3, KOH, tBuOK). The resultant polymeric materials show significantly enhanced basicity, while maintaining the original mesoporous structure. It was then used as a heterogeneous base catalyst for Knoevenagel condensations and transesterification reactions. Catalytic results show that the deprotonated mpg-C3N4 exhibits high efficiency for promoting these reactions as a solid and polymeric catalyst. The reactions between various aldehydes and malonic derivatives, alcohols and β-ketoesters could proceed smoothly in the presence of deprotonated mpg-C3N4. The catalyst could be recycled and reused for several runs without any loss of intrinsic catalytic activity.
Introduction
Chemical Transformations promoted by the base catalysis are important industrial processes for fine chemical synthesis.1 Carbon–carbon bond formation reactions, such as Aldol condensations, Knoevenagel condensations and transesterification reactions, are of special importance.2 Conventionally, stoichiometric amounts of homogeneous bases, such as hydroxides and methoxides, are required in many organic reactions, which produce large amounts of metal salts as byproducts.3 Owing to the increasing attention towards the development of green and sustainable heterogeneous catalysis, it is highly desirable to develop more sustainable, efficient and economic heterogeneous solid catalysts,4 affording high yields and selectivities towards the desirable products and avoiding the use of noxious substances, along with little or no unwanted byproducts.Over the past few years, research on solid-base catalysts, such as hydrotalcites,5 apatites,6 metal oxides,7 clays, metal–organic materials8 and alkali doped zeolites,9 has increased rapidly. However, one reason for the limited use of these catalysts arises from the drawbacks such as their instability and partial dissolution into the reaction media. There is an urgent need for the development of new and stable basic solid materials. Nanostructured carbons have been widely employed as versatile catalyst (support) due to their unique physical and chemical properties such as thermal stability and inertness to various solvents.10 The incorporation of nitrogen atoms in the texture of polymers and carbons gives rise to the basic function which dictates the basic catalytic performance of the materials.11 Thomas et al. found that mesoporous poly(benzimidazole) could actually act as a catalyst for the Knoevenagel condensation.12 Vinu et al. reported that carbon nitride nanoparticles with high nitrogen content exhibited great catalytic performance for the transesterification of β-keto esters with various alcohols.13 Bitter et al. reported the preparation of basic nitrogen containing carbon nanotubes and related their good catalytic activity in Knoevenagel condensations with the amount of pyridinic nitrogen incorporated in the structure.11 Most recently, Park and coworkers demonstrated the use of high nitrogen containing mesoporous carbon nitride as base catalyst for Knoevenagel condensation of ethylcyanoacetate with aromatic aldehydes under the assistance of microwave.14
Recently, we have shown that the organic solid-state material mpg-C3N4 is an alternative (photo)catalyst complementing metal oxides and carbon due to its unique electronic properties and large surface area.15 This two-dimensional π-conjugated polymer features a semiconductor band gap of 2.7 eV with suitable band position to activate photoredox reactions of H2O and O2, achieving water splitting, alcohol oxidation, and amine oxidative coupling reactions. However, the surface basic functions of mpg-C3N4 are relatively less explored in organocatalysis application. mpg-C3N4 is typically prepared by the direct thermal condensation of nitrogen containing precursors (cyanamide, dicyandiamide) on a nanosized silica template. The template is subsequently removed by ammoniumbifluoride to obtain porous frameworks and also to create surface defects of terminal amino groups and of bridging nitrogens, associated with incomplete condensation. As in graphite, the surface defects are believed to promote electron relocalization on the surface (Note: in perfect carbon nitride sheets, there is no electron localization in the π state, thus no active basicity), thus inducing Lewis-base character on mpg-C3N4 towards metal-free coordination chemistry and catalysis (Fig. 1). It is therefore believed that mpg-C3N4 is able to promote base-catalyzed organic reactions,16 like C–C coupling and C–H activation reactions. Indeed, our previous studies17 and others18 have applied the Lewis-base character of mpg-C3N4 in the chemical binding and activation of carbon dioxide.
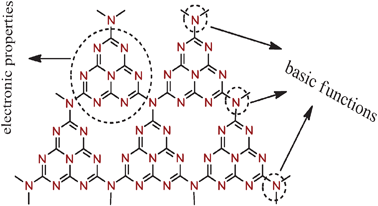 | ||
| Fig. 1 Multifunctional properties of graphitic carbon nitride. | ||
Here, we extend the application of mpg-C3N4 catalysts for promoting C–C coupling reactions, as archetypically exemplified by Knoevenagel and transesterification reactions. This study is meant to be a first step towards enriching the content of carbon nitride chemistry by potentially integrating the base catalysis into photoredox catalysis of carbon nitride semiconductor, achieving valuable cascade reactions in heterogeneous organic (photo)synthesis.
Results and discussion
Firstly, the Knoevenagel condensation between benzaldehyde and malononitrile was selected as the probe reaction to determine the catalytic activity of various carbon nitrides and to optimize the reaction conditions. The results are summarized in Table 1. The blank reaction between benzaldehyde and malononitrile at 70 °C in CH3CN solvent only resulted in the formation of 7% benzoic acid, which originated from the auto-oxidation of benzaldehyde in air. Using g-C3N4 as the catalyst, the conversion increased to 19% together with an unsatisfactory selectivity of 30% (entry 2). In the presence of mpg-C3N4, higher conversion of 33% and selectivity of 74% (entry 3) were achieved due to its well-connected mesopores which facilitated good mass transfer diffusion and the access to more basic sites. However, the catalytic activity of the as-synthesized mpg-C3N4 was still unsatisfactory, and thus further modification or post-functionalization was applied to improve the catalytic efficiency. Direct deprotonation of the catalyst seems to be the potential strategy to improve its performance in the condensation reaction as the use of aqueous NH4HF2 (during the synthesis of mpg-C3N4) might protonate the surface N groups. Indeed, the treatment of mpg-C3N4 at elevated temperatures, which are believed to facilitate the deprotonation, could lead to a significantly enhanced catalytic activity (entries 5–7).19 However, increasing temperature to 400 °C brought about abrupt decrease of conversion, which was attributed to the structural etching and pore shrinkage at such a high temperature (see Fig. 2).| Entry | Catalysts | Con. (%) | Sel. (%) |
|---|---|---|---|
| a Reaction performed at 70 °C for 2 h using CH3CN (10 ml) as the solvent, 1 mmol aldehyde, 2 mmol nitrile, 50 mg catalyst. b mpg-C3N4 (–CO3, –OH and –tBu) refer to mpg-C3N4 treated with K2CO3, KOH and tBuOK solutions respectively. c 1 mmol nitrile. d Ar atmosphere. | |||
| 1 | / | 7 | / |
| 2 | g-C3N4 | 19 | 30 |
| 3 | mpg-C3N4 | 33 | 74 |
| 4 | mpg-C3N4-H | 23 | 58 |
| 5 | mpg-C3N4-200 oC | 35 | 74 |
| 6 | mpg-C3N4-300 oC | 57 | 89 |
| 7 | mpg-C3N4-400 oC | 39 | 89 |
| 8b | mpg-C3N4-CO3 | 66 | 93 |
| 9b | mpg-C3N4-OH | 71 | 93 |
| 10b | mpg-C3N4-tBu | 80 | 96 |
| 11c | mpg-C3N4-tBu | 67 | 94 |
| 12d | mpg-C3N4-tBu | 89 | 98 |
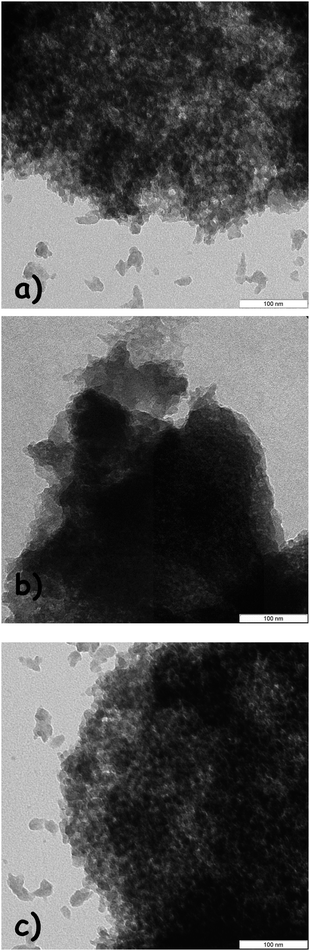 | ||
| Fig. 2 TEM images of (a) mpg-C3N4, (b) mpg-C3N4-400 °C and (c) mpg-C3N4-tBu. | ||
On the contrary, protonated mpg-C3N4 upon immersing the catalyst in HCl solution (0.1 M) proves the concept, giving worse results (entry 4). Another possible and even more practical approach for the deprotonation is the treatment with base solution. As a result, substantial increase in catalytic activity of mpg-C3N4 was observed after the basic treatment (0.1 M), in the order tBuOK > KOH > K2CO3. Using mpg-C3N4-tBu as the activator of the catalyst, a conversion of benzaldehyde of 80% with 96% selectivity towards the target product has been realized. The structure and mesoporosity of mpg-C3N4 are well kept after the treatment with tBuOK solution, which can be clearly seen from the TEM image (Fig. 2c). No evident changes in terms of local structure after the treatment are seen.20 Therefore in a series of experiments below, we would employ the mpg-C3N4-tBu as the catalyst, which was deprotonated by tBuOK solution. It was expected that decreasing the malononitrile/benzaldehyde molar ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in a significant drop in the reaction rate, and only 67% conversion was achieved for the reaction using 1 equiv. of malononitrile (entry 11). When the reaction was carried out under an Ar atmosphere, a higher conversion (89%) was observed since a minor amount of the oxidative byproduct of benzoic acid was generated (entry 12), which could strongly adhere to the surface of mpg-C3N4 and deactivate the basic sites.
1 resulted in a significant drop in the reaction rate, and only 67% conversion was achieved for the reaction using 1 equiv. of malononitrile (entry 11). When the reaction was carried out under an Ar atmosphere, a higher conversion (89%) was observed since a minor amount of the oxidative byproduct of benzoic acid was generated (entry 12), which could strongly adhere to the surface of mpg-C3N4 and deactivate the basic sites.
To rule out the potential leaching problem for this heterogeneous catalyst, the condensation of benzaldehyde with malononitrile as a model reaction was carried out with mpg-C3N4-tBu under the identical conditions of entry 11 in Table 1. The catalyst was filtered at around 50% conversion at the reaction temperature and the reaction was again performed with the filtrate under the same conditions. As shown in Fig. 3, the reaction was completely stopped by the removal of the catalyst. Furthermore, to exclude the possibility that the adsorbed base (tBuOK) on the surface of the catalyst may contribute to the enhanced catalytic performance, inductive coupled plasma (ICP-OES) measurement was carried out after dissolving mpg-C3N4-tBu in nitric acid solution. It turned out that we could not detect the content of K on the mpg-C3N4-tBu catalyst, which indicated that the base such as tBuOK was thoroughly washed and thus made no contribution for the catalysis.
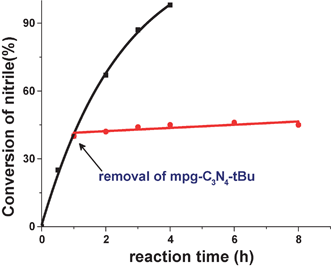 | ||
| Fig. 3 Effect of removal of catalyst on the condensation reaction between benzaldehyde and malononitrile. The arrow indicates the removal of mpg-C3N4-tBu. | ||
The influence of the amount of catalyst on the reaction was also studied, as shown in Fig. 4. As expected, increasing the amount of mpg-C3N4-tBu from 30 mg to 70 mg could lead to a significant enhancement of the conversion (from 15% to 50%). Note that the selectivity was meanwhile largely influenced by the catalyst amount, and increasing the catalyst concentration would give higher selectivity. The phenomenon could be attributed to the suppression of the byproduct of benzoic acid with the increase in basicity of the reaction system.
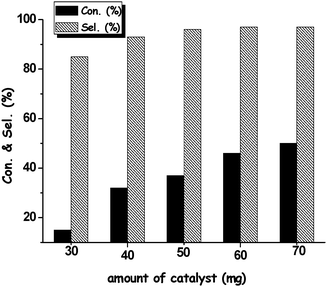 | ||
| Fig. 4 Effect of the amount of mpg-C3N4-tBu catalyst on the condensation of 4-methyl benzaldehyde and malononitrile. Reaction conditions: 50 mg mpg-C3N4-tBu, 1 mmol aldehyde, 1 mmol nitrile, 5 ml CH3CN, 70 °C, 1 h. | ||
In order to demonstrate the versatility of mpg-C3N4 for Knoevenagel condensations, we submitted various aldehydes to malononitrile or ethyl cyanoacetate with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of mpg-C3N4-tBu, and the results are listed in Table 2. The reactions between aromatic aldehydes, such as benzaldehyde, 4-methyl-, 4-methoxy- and 2-chlorobenzaldehyde, and malononitrile afforded high yields of the corresponding Knoevenagel condensation products (entries 1, 4–7). The condensation reaction between benzaldehyde and malononitrile could even proceed under room temperature, although a much longer reaction time was needed to realize the high conversion of the substrates (entry 3). The catalyst was also active when furfural was used as one of the substrates. The results were similar to the other aromatic aldehydes, and no byproduct as well as an excellent selectivity towards the target compound was observed (entry 8). Aliphatic aldehyde, such as hexanal, could also undergo condensation with malononitrile to afford the final product with high efficiency (entry 9). However, when cyclohexanone was used as the starting material, only a relatively low reaction rate was observed (entry 10). It is well known that ketones are generally less reactive for the condensation, relative to aldehydes.21 A dramatic drop in terms of conversion was observed when ethyl cyanoacetate instead of malononitrile was used (entry 5), while diethyl malonate did not even react with benzaldehyde in the present reaction (entry 2), indicating the relatively low basicity of mpg-C3N4-tBu.
1 in the presence of mpg-C3N4-tBu, and the results are listed in Table 2. The reactions between aromatic aldehydes, such as benzaldehyde, 4-methyl-, 4-methoxy- and 2-chlorobenzaldehyde, and malononitrile afforded high yields of the corresponding Knoevenagel condensation products (entries 1, 4–7). The condensation reaction between benzaldehyde and malononitrile could even proceed under room temperature, although a much longer reaction time was needed to realize the high conversion of the substrates (entry 3). The catalyst was also active when furfural was used as one of the substrates. The results were similar to the other aromatic aldehydes, and no byproduct as well as an excellent selectivity towards the target compound was observed (entry 8). Aliphatic aldehyde, such as hexanal, could also undergo condensation with malononitrile to afford the final product with high efficiency (entry 9). However, when cyclohexanone was used as the starting material, only a relatively low reaction rate was observed (entry 10). It is well known that ketones are generally less reactive for the condensation, relative to aldehydes.21 A dramatic drop in terms of conversion was observed when ethyl cyanoacetate instead of malononitrile was used (entry 5), while diethyl malonate did not even react with benzaldehyde in the present reaction (entry 2), indicating the relatively low basicity of mpg-C3N4-tBu.
| Entry | Aldehydes | Nitriles | t/h | Con. (Sel.)/% |
|---|---|---|---|---|
| a Reaction performed at 70 °C using CH3CN (10 ml) as the solvent, 1 mmol aldehyde (ketone), 1 mmol nitrile, 50 mg mpg-C3N4-tBu. b Room temperature. | ||||
| 1 |

|

|
4 | 99 (97) |
| 2 |

|

|
4 | /(/) |
| 3b |

|

|
18 | 93 (96) |
| 4 |

|

|
4.5 | 85 (100) |
| 5 |

|
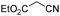
|
4 | 5 (100) |
| 6 |

|

|
5 | 71 (100) |
| 7 |

|

|
5 | 95 (100) |
| 8 |

|

|
5 | 96 (100) |
| 9 |

|

|
5 | 72 (91) |
| 10 |

|

|
19 | 30 (100) |
The catalyst is stable and can be reused after the reaction. The used mpg-C3N4-tBu was separated and cleaned, and then applied in the next run under the same conditions. Fig. 5 shows the comparison of the activity of mpg-C3N4-tBu catalyst during 5 consecutive uses. Upon recycling, mpg-C3N4-tBu virtually maintained its original activity without appreciable decay in the conversion. There was a dramatic decrease of conversion in the second run. However, a subsequent treatment of the catalyst with a 0.1 M aqueous solution of KOH allowed recovery of the catalytic activity of mpg-C3N4-tBu. Apparently, the basic treatment could remove small amounts of benzoic acid and other acids (as CO2) on the catalyst surface that ultimately led to catalyst poisoning.
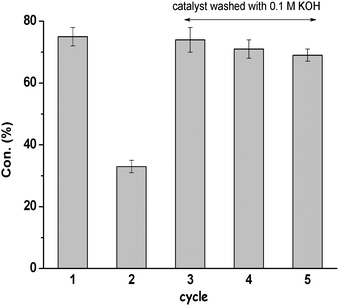 | ||
| Fig. 5 Evolution of Knoevenagel condensation between 4-methyl benzaldehyde and malononitrile during five consecutive catalytic uses with mpg-C3N4-tBu. | ||
In addition to Knoevenagel condensations, mpg-C3N4-tBu could also be used as an active base catalyst for transesterification reactions. The reactions of a variety of primary, allylic, cyclic and aliphatic alcohols with ethyl acetoacetate were carried out using mpg-C3N4-tBu at 110 °C. As can be seen from Table 3, ethyl acetoacetate is successfully transesterfied into the corresponding esters in the presence of various alcohols. Transesterification of allylic alcohols is difficult since the product readily undergoes decarboxylative rearrangement. However, in our catalytic system, unsaturated allylic alcohol such as cinnamyl alcohol underwent transesterification affording ester in high yield (entry 3). Long-chain primary alcohol (entry 4) and cyclic, hindered alcohol (entry 5) could both be converted into the corresponding esters; however, the reaction of the former proceeded much more smoothly than that of the latter.
| Entry | Alcohols | β-Ketoester | t/h | Con. (Sel.)/% |
|---|---|---|---|---|
| a Reaction performed at 110 °C using toluene (2 ml) as the solvent, 1 mmol alcohol, 2 mmol ethyl acetoacetate and 50 mg mpg-C3N4-tBu. | ||||
| 1 |

|

|
4.5 | 88 (96) |
| 2 |

|

|
6 | 61 (99) |
| 3 |

|

|
5 | 90 (97) |
| 4 |

|

|
5 | 98 (99) |
| 5 |

|

|
5.5 | 48 (99) |
Conclusion
In this contribution, due to the basic properties of mpg-C3N4, we used it as a polymeric solid base catalyst. The catalytic performance could be significantly improved if the catalyst was pre-deprotonated with basic solution. During the catalytic tests in Knoevenagel condensations and transesterification reactions, the catalyst was active and afforded high yields of the corresponding products, respectively. The heterogeneous catalyst was also highly stable and could be recycled several times without loss of intrinsic catalytic activity.The results provide experimental evidence of basic properties of graphitic carbon nitride, which demonstrates the multifunctional catalytic nature of graphitic carbon nitride. It is anticipated that mpg-C3N4 would be a multifunctional oxidation-base catalyst for the cascade process, which opens up new possibilities for increased safety as well as manipulation of equilibrium. Therefore we expect that carbon nitride and its derivatives could be further used as multifunctional catalyst in a variety of functional transformations.
Experimental section
Catalyst preparation
Characterization
TEM images were recorded on an Omega 912 transmission electron microscope. XRD measurements were carried out in reflection mode (CuKα radiation) on a Bruker D8 Advance diffractometer.Catalytic test
The catalytic activity of mpg-C3N4 was tested for Knoevenagel condensation and transesterification reactions using a round bottomed flask equipped with a cooling condenser. For the former reaction, benzaldehyde 1 mmol, malononitrile 1 mmol, solvent acetonitrile 5 ml, catalyst 50 mg, temperature 70 °C; for the latter, ethyl acetate 2 mmol, ethanol 1 mmol, catalyst 50 mg, solvent toluene 2 ml, temperature 110 °C.The results were obtained and recorded using a HP6890 series. Injections were done using an Agilent autosampler 7683B. The GC conditions for the analysis were as follows: He as the carrier gas with a flow rate of 1.5 ml min−1, injection port temperature 250 °C, 1 min at 50 °C then a ramp to 300 °C with the rate of 30 °C min−1 and kept at 300 °C for another 1 min.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21033003 and 21173043) and the Max Planck Society.Notes and references
- (a) K. Tanabe and W. F. Holderich, Appl. Catal., A, 1999, 181, 3523 CrossRef; (b) M. J. Climent, A. Corma, R. Guil-Lopez, S. Iborra and J. Primo, Catal. Lett., 1999, 59, 33 CrossRef CAS; (c) Y. Ono, J. Catal., 2003, 216, 405 CrossRef.
- (a) R. A. Sheldon, J. Mol. Catal. A: Chem., 1996, 107, 75 CrossRef CAS; (b) A. Zapf and M. Beller, Top. Catal., 2002, 19, 101 CrossRef CAS.
- (a) H. U. Blaser, Catal. Today, 2000, 60, 161 CrossRef CAS; (b) G. J. Kelly, F. King and M. Kett, Green Chem., 2002, 4, 392 RSC.
- F. Winter, V. Koot, A. J. van Dillen, J. W. Geus and K. P. de Jong, J. Catal., 2005, 236, 91 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra and A. Velty, J. Catal., 2004, 221, 474 CrossRef CAS.
- S. Sebti, A. Solhy, R. Tahir and A. Smahi, Appl. Catal., A, 2002, 235, 273 CrossRef CAS.
- Z. Liu, J. A. Cortes-Concepcion, M. Mustian and M. D. Amiridis, Appl. Catal., A, 2006, 302, 232 CrossRef CAS.
- X. G. Wang, Y. H. Tseng, J. C. C. Chan and S. F. Cheng, J. Catal., 2005, 233, 266 CrossRef CAS.
- Y. Goa, P. Wu and T. Tasumi, J. Catal., 2004, 224, 107 CrossRef CAS.
- (a) P. Serp, m. Corrias and P. Kalck, Appl. Catal., A, 2003, 253, 337 CrossRef CAS; (b) R. Arrigo, M. Havecker, S. Wrabetz, R. Blume, M. Lerch, J. McGregor, E. P. J. Parrott, J. A. Zeitler, L. F. Gladden, A. Knop-Gericke, R. Schlogl and D. S. Su, J. Am. Chem. Soc., 2010, 132, 9616 CrossRef CAS; (c) J. P. Tessonnier, A. Villa, O. Majoulet, D. S. Su and R. Schlogl, Angew. Chem., Int. Ed., 2009, 48, 6543 CrossRef CAS.
- S. Van Dommele, K. P. de Jong and J. H. Bitter, Top. Catal., 2009, 52, 1575 CrossRef.
- P. Makowski, J. Weber, A. Thomas and F. Goettmann, Catal. Commun., 2008, 10, 243 CrossRef CAS.
- X. Jin, V. V. Babasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884 CrossRef CAS.
- M. B. Ansari, H. L. Jin, M. N. Parvin and S. E. Park, Catal. Today, 2011 DOI:10.1016/j.cattod.2011.07.024.
- (a) F. Z. Su, S. C. Mathew, G. Lipner, X. Z. Fu, M. Antonietti, S. Blechert and X. C. Wang, J. Am. Chem. Soc., 2010, 132, 16299 CrossRef CAS; (b) F. Z. Su, S. C. Mathew, L. Mohlmann, M. Antonietti, X. C. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657 CrossRef CAS; (c) Y. Wang, H. R. Wang, J. Yao, X. C. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446 RSC.
- A. Thomas, A. Fischer, F. Geottmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- F. Goettmann, A. Thomas and M. Antonietti, Angew. Chem., Int. Ed., 2007, 46, 2717 CrossRef CAS.
- M. B. Ansari, B. H. Min, Y. H. Mo and S. E. Park, Green Chem., 2011, 13, 1416 RSC.
- Y. J. Zhang, A. Thomas, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 50 CrossRef CAS.
- See ESI†.
- B. M. Choudary, M. L. Kantam, P. Sreekanth, T. Bandopadhyay, F. Figueras and A. Tuel, J. Mol. Catal. A: Chem., 1999, 142, 361 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns. See DOI: 10.1039/c2cy00012a |
| This journal is © The Royal Society of Chemistry 2012 |