Synthesis of xanthene derivatives by employing Fe3O4 nanoparticles as an effective and magnetically recoverable catalyst in water†
Bahador
Karami
*a,
S. Jafar
Hoseini
a,
Khalil
Eskandari
a,
Abdolmohammad
Ghasemi
b and
Hassan
Nasrabadi
a
aDepartment of Chemistry, Yasouj University, Yasouj, Iran. E-mail: karami@mail.yu.ac.ir; Fax: +98 7412224167; Tel: +98 7412223048
bIslamic Azad University, Gachsaran Branch, Gachsaran, Iran
First published on 27th October 2011
Abstract
An efficient and environmentally adapted synthesis of xanthene derivatives by condensation of a wide range of aryl aldehydes and 1,3-cyclohexanediones in water using a catalytic amount of Fe3O4 nanoparticles is explained. The Fe3O4 nanoparticles were characterized by powder X-ray diffraction (XRD), transmission electron microscopy (TEM) and FT-IR spectroscopy.
Introduction
Xanthene and its derivatives have received significant attention in recent years due to their wide range of biological and therapeutic properties.1,2 The importance of xanthene derivatives clearly was realized from their usage as dyes,3 sensitizers in photodynamic therapy for destroying the tumor cells,4 pH-sensitive fluorescent materials for visualization of biomolecules,5 and in laser technologies.6 Furthermore, some of the xanthene based compounds have found applications as antagonists for paralyzing the action of zoxalamine and in photodynamic therapy.7Several polycyclic compounds containing the xanthene skeleton are isolated from natural sources.8 Xanthene and its derivatives are prepared by different methods, including the reaction of aryloxymagnesium halides with triethylorthoformate,9 cyclodehydration,10 trapping of benzynes by phenols,11 intramolecular phenyl carbonyl coupling reactions of benzaldehydes and acetophenones,12 and cyclocondensation between 2-hydroxy aromatic aldehydes and 2-tetralone.13 In view of the importance of xanthene derivatives, many methods for the synthesis of these compounds were reported including condensation of β-naphthol and aldehydes or acetals catalyzed by silica sulfuric acid, HCl/CH3COOH or H3PO4.14 However some of these methods involved long reaction times, and unsatisfactory yields. Therefore improvements in these syntheses have been sought continuously. In this work, preparation, high activation, and regeneration of Fe3O4 nanoparticles as a catalyst in organic synthesis have been shown.
A new method for the synthesis of 1,8-dioxo-octahydroxanthenes was obtained by condensation of 1,3-cyclohexanediones and benzaldehydes in the presence of magnetic nanoparticles Fe3O4 as an effective catalyst in water as a solvent (Scheme 1).
 | ||
| Scheme 1 Synthesis of 1,8-dioxo-octahydroxanthenes in the presence of 1,3-cyclohexanediones, benzaldehydes and magnetic nanoparticles Fe3O4. | ||
The synthesis mechanism of 1,8-dioxo-octahydroxanthene derivatives has been shown in Scheme 2.
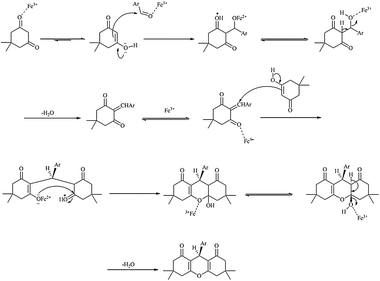 | ||
| Scheme 2 The suggested mechanism for synthesis of 1,8-dioxo-octahydroxanthene derivatives. | ||
Experimental
Transmission electron microscopy (TEM) studies of the nanostructures were carried out with a JEOL JEM 3010 instrument operating at an accelerating voltage of 300 kV. X-Ray diffraction (XRD, D8, Advance, Bruker, AXS) patterns were obtained for characterization of the heterogeneous catalyst. Melting points were measured on an electrothermal KSB1N apparatus. IR spectra were recorded in the matrix of KBr with a JASCO FT-IR-680 plus spectrometer. 1H NMR and 13C NMR spectra were recorded on a FT-NMR Bruker Avance Ultra Shield Spectrometer at 400.13 and 100.62 MHz in CDCl3 as solvent in the presence of tetramethylsilane as internal standard. TLC was performed on TLC-Grade silica gel-G/UV 254 nm plates. The products were isolated and characterized by physical and spectral data and they were compared with authentic samples (Table 1).9,10,15–26| Entry | Aldehyde 2 | Producta4 | Time (min) | Yieldb (%) | Mp (°C)/(lit.) |
|---|---|---|---|---|---|
| a Identified by comparison with authentic samples. b Refers to isolated yields. | |||||
| 1 |

|

|
8 | 96 | 202–204 (201–202)15 |
| 2 |

|

|
33 | 90 | 230–232 (230–232)15 |
| 3 |

|

|
53 | 88 | 215–217 (216–217)16 |
| 4 |

|

|
6 | 95 | 219–221 (221–223)16 |
| 5 |

|

|
42 | 89 | 226–227 (226–228)17 |
| 6 |

|

|
14 | 93 | 209–211 (210–212)18 |
| 7 |

|

|
19 | 92 | 223–225 (224–226)17 |
| 8 |

|

|
34 | 90 | 250–252 (249–252)19 |
| 9 |

|

|
40 | 86 | 189–191 (190–191)20 |
| 10 |

|

|
37 | 88 | 238–239 (236–239)10 |
| 11 |

|

|
16 | 90 | 245–247 (> 300)21 |
| 12 |

|

|
23 | 86 | 238–240 (236–238)22 |
| 13 |

|

|
10 | 95 | 271–273 (272–273)23 |
| 14 |

|

|
62 | 89 | 260–262 (262–263)15 |
| 15 |

|

|
9 | 92 | 224–227 (224–226)24 |
| 16 |

|

|
42 | 88 | 250–252 (249–252)9 |
| 17 |

|

|
45 | 86 | 170–172 (169–171)25 |
| 18 |

|

|
21 | 92 | 252–25526 |
General procedure for preparation of Fe3O4 nanoparticles
To prepare Fe3O4 nanoparticles, 5.2 g of FeCl3 and 2.0 g of FeCl2 were successively dissolved in 25 mL of distilled water containing 0.85 mL of 12.1 N HCl. The resulting solution was added dropwise into 250 mL of 1.5 M NaOH solution under vigorous stirring. The last step generated an instant black precipitate. The precipitate was isolated in the magnetic field, and the supernatant was removed from the precipitate by decantation.27General procedure for a facile and rapid synthesis of xanthene derivatives in water by Fe3O4 nanoparticles as a new, highly efficient, and reusable catalyst
A mixture of aromatic aldehydes (1 mmol), 1,3-cyclohexanediones (2 mmol) and magnetic nanoparticles Fe3O4 (0.01 mmol) in H2O (5mL) was stirred at 80 °C in the oil bath for the time indicated in Table 1. The progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature after completion of reaction. The solid material was filtered, and washed with water (2 × 10 mL) to furnish nearly net products. The products were further purified by recrystallization from ethanol, and thereupon the catalyst was isolated from the pure product. All products were deduced from their IR and NMR spectroscopic data, and their melting points were compared with authentic samples.Reusability of the catalyst
At the end of the reaction, the catalyst was filtered, washed with diethyl ether, dried at 130 °C for 1 h, and reused in another reaction. We found that magnetic nanoparticles Fe3O4 showed high catalytic activity with very short reaction times. Moreover, they can be recovered and reused several times without significant loss of activity. The results of these observations for the model reaction are shown in Table 2 and Fig. 1.| Product | Total reusability | Yield (%) | Time (min) |
|---|---|---|---|

|
1 | 96 | 8 |
| 2 | 95 | 8 | |
| 3 | 95 | 9 | |
| 4 | 97 | 10 | |
| 5 | 97 | 9 |
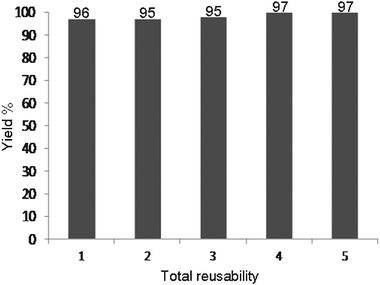 | ||
| Fig. 1 The reusability of the catalyst in the synthesis of 3,3,6,6-tetramethyl-9-phenyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (Table 1, entry 1). | ||
Representative spectral data
Table 1, entry 1: mp, 202–204 °C; IR (KBr) (νmax, cm−1): 3060, 2958, 1661, 1624, 1468, 1199, 742, 700; 1H NMR (400 MHz, CDCl3); δH (ppm): 0.79 (6H, s, 2 × CH3), 0.90 (6H, s, 2 × CH3), 2.00 (4H, dd, 2 × CH2, 1J = 16.4 Hz, 4J = 28.8 Hz), 2.27 (4H, s, 2 × CH2), 4.55 (1H, s, CH), 6.90–7.10 (5H, m, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 28.47, 30.44, 32.97, 33.36, 42.00, 51.88, 116.79, 127.51, 129.19,129.52, 145.25, 163.42, 196.66.Table 1, entry 6: mp, 209–211 °C; IR (KBr) (νmax, cm−1): 3050, 2995, 1660, 1620, 1480, 1375, 1188, 1090, 845; 1H NMR (400 MHz, CDCl3); δH (ppm): 1.04 (6H, s, 2 × CH3), 1.12 (6H, s, 2 × CH3), 2.24 (4H, s, 2 × CH2), 2.47 (4H, s, 2 × CH2), 3.78 (3H, s, OCH3), 3.81 (6H, s, 2 × OCH3), 4.72 (1H, s, CH), 6.52 (2H, s, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 27.18, 29.36, 31.80, 32.17, 40.90, 50.75, 56.09, 60.68, 105.75, 115.57, 136.60, 139.73, 152.79, 162.34, 196.45.
Table 1, entry 7: mp, 223–225 °C; IR (KBr) (νmax, cm−1): 3040, 2990, 2970, 1660, 1620, 1500, 1360, 1200, 1160, 1180; 1H NMR (400 MHz, CDCl3); δH (ppm): 1.00 (6H, s, 2 × CH3), 1.11 (6H, s, 2 × CH3), 2.21 (4H, q, J = 16.4 Hz, 2 × CH2), 2.47 (4H, s, 2 × CH2), 4.73 (1H, s, CH), 6.91 (2H, m, Ar-H), 7.27 (2H, m, Ar-H); 13C NMR (100 MHZ, CDCl3); δC (ppm): 27.28, 29.26, 32.19, 40.84, 50.73, 114.71, 114.93, 115.49, 129.88, 139.99, 160.15, 162.58, 196.34.
Table 1, entry 9: mp, 189–191 °C; IR (KBr) (νmax, cm−1): 3065, 2960, 2880, 1660, 1615, 1450, 1375, 1200, 1160, 1138; 1H NMR (400 MHZ, CDCl3); δH (ppm): 1.01 (6H, s, 2 × CH3), 1.10 (6H, s, 2 × CH3), 1.18 (6H, d, J = 5.2 Hz, 2 × CH3), 2.21 (4H, m, 2 × CH2), 2.46 (4H, s, 2 × CH2), 2.79 (1H, bb, CH), 4.73 (1H, s, CH), 7.05 (2H, d, J = 6.8 Hz, Ar-H), 7.19 (2H, m, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 23.90, 27.49, 29.21, 31.30, 32.21, 33.60, 40.90, 50.80, 126.12, 128.12, 141.39, 146.51, 162.15, 196.46.
Table 1, entry 11: mp, 245–247 °C; IR (KBr) (νmax, cm−1): 3040, 2957, 1666, 1620, 1462, 1425, 1365, 1200, 1162, 1003, 808; 1H NMR (400 MHz, CDCl3); δH (ppm): 0.97 (12H, s, 4 × CH3), 1.07 (12H, s, 4 × CH3), 2.18 (8H, s, 4 × CH2), 2.44 (8H, dd, 1J = 36.4 Hz, 4J = 17.6 Hz, 4 × CH2), 4.71 (2H, s, 2 × CH), 7.08 (2H, s, Ar-H), 7.27 (2H, s, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 25.01, 27.66, 28.98, 30.74, 32.24, 40.85, 50.64, 115.70, 127.88, 141.74, 162.42, 196.36.
Table 1, entry 12: mp, 238–240 °C; IR (KBr) (νmax, cm−1): 3095, 2957, 1659, 1629, 1462, 1203, 1158, 769; 1H NMR (400 MHz, CDCl3); δH (ppm): 1.03 (12H, s, 4 × CH3), 1.08 (12H, s, 4 × CH3), 2.15 (8H, dd, 2J = 24 Hz, 4J = 16 Hz, 4 × CH2), 2.48 (8H, dd, 2J = 45.2 Hz, 4J = 17.6 Hz, 4 × CH2), 4.72 (2H, s, 2 × CH), 7.07–7.09 (3H, m, Ar-H), 7.15 (1H, s, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 28.02, 29.57, 31.76, 32.56, 41.27, 51.27, 116.01, 126.84, 128.18, 144.04, 162.72, 196.66.
Table 1, entry 14: mp, 260–262 °C; IR (KBr) (νmax, cm−1): 3050, 2955, 1658, 1616, 1467, 1175, 1126, 827; 1H NMR (400 MHz, CDCl3); δH (ppm): 2.01 (4H, m, 2 × CH2), 2.26 (3H, s, CH3), 2.35 (4H, m, 2 × CH2), 2.59 (4H, m, 2 × CH2), 4.78 (1H, s, CH), 7.03 (2H, d, J = 7.2 Hz, Ar-H), 7.19 (2H, d, J = 7.2 Hz, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 20.31, 21.07, 27.15, 31.22, 36.99, 117.00, 128.25, 128.83, 135.85, 141.56, 163.84, 196.56.
Table 1, entry 15: mp, 224–227 °C; IR (KBr) (νmax, cm−1): 3070, 2950, 1664, 1617, 1467, 1172, 830; 1H NMR (400 MHz, CDCl3); δH (ppm): 2.07 (4H, m, 2 × CH2), 2.35 (4H, m, 2 × CH2), 2.61 (4H, m, 2 × CH2), 4.88 (1H, s, CH), 7.48 (2H, d, J = 8.8 Hz, Ar-H), 8.10 (2H, d, J = 8.8 Hz, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 20.22, 27.14, 32.23, 36.81, 115.70, 123.41, 129.42, 145.48, 151.73, 164.60, 196.45.
Table 1, entry 16: mp, 250–252 °C; IR (KBr) (νmax, cm−1): 3095, 2960, 1619, 1563, 1463, 1367, 1290, 1215, 1180, 1086, 1033, 1005, 814, 650; 1H NMR (400 MHz, CDCl3); δH (ppm): 1.86 (2H, s, CH2), 2.05 (3H, t, J = 12.4 Hz, CH2), 2.15 (1H, d, J = 8.4 Hz, CH2), 2.43 (2H, m, CH2), 2.57 (3H, t, J = 19.21 Hz, CH2), 2.75 (1H, d, J = 8.8 Hz, CH2), 4.58 (1H, s, CH), 6.91 (1H, d, J = 4.4 Hz, Ar-H), 7.13 (1H, s, Ar-H), 7.26 (1H, d, J = 4.4 Hz, Ar-H), 10.77 (1H, s, OH); 13C NMR (100 MHz, CDCl3); δC (ppm): 19.54, 19.87, 27.89, 27.97, 29.72, 35.95, 36.95, 112.02, 117.00, 117.26, 119.39, 126.92, 130.46, 130.68, 150.03, 170.73, 173.37, 197.09, 201.31.
Table 1, entry 17: mp, 170–172 °C; IR (KBr) (νmax, cm−1): 3050, 2990, 1660, 1620, 1450, 1200, 1130, 828; 1H NMR (400 MHz, CDCl3); δH (ppm): 1.19 (6H, d, J = 6.8 Hz, 2 × CH3), 2.01 (4H, m, 2 × CH2), 2.34 (4H, m, 2 × CH2), 2.61 (4H, m, 2 × CH2), 2.81 (1H, t, J = 7.2 Hz, CH), 4.80 (1H, s, CH), 7.06 (2H, d, J = 8 Hz, Ar-H), 7.19 (2H, d, J = 8 Hz, Ar-H); 13C NMR (100 MHz, CDCl3); δC (ppm): 20.28, 23.92, 27.15, 31.04, 33.59, 36.99, 117.03, 126.16, 146.52, 163.95, 196.64.
Results and discussion
Fig. 2 shows the XRD patterns of Fe3O4 nanoparticles. A number of prominent Bragg reflections by their indices ((220), (311), (400), (422), (511) and (440)) reveal that the resultant nanoparticles were Fe3O4 with a spinel structure.28 The size of the Fe3O4 nanoparticles was also determined from X-ray line broadening using the Debye–Scherrer formula given as D = 0.9λ/βcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where D is the average crystalline size (Å), λ the X-ray wavelength used (nm), β the angular line width at half maximum intensity (radians) and θ the Braggs angle (degrees). For (311) reflection at 2θ ≈ 36°, for β = 0.011 radians (0.64°), λ = 1.54 Å and θ ≈ 18°, the average size of the Fe3O4 nanoparticles is estimated to be around 13 nm. Transmission electron microscopy (TEM) analyses were used for characterization (Fig. 3). The TEM image reveals the spherical Fe3O4 nanoparticles with the average size of 10–20 nm, consistent with average size obtained from the Debye–Scherrer formula. The FT-IR spectra of Fe3O4 nanoparticles are shown in Fig. 4. The absorbance bands at 584.3 cm−1 ascribed to Fe+2 O−2 are consistent with the reported IR spectra for spinel Fe3O4.29,30
θ, where D is the average crystalline size (Å), λ the X-ray wavelength used (nm), β the angular line width at half maximum intensity (radians) and θ the Braggs angle (degrees). For (311) reflection at 2θ ≈ 36°, for β = 0.011 radians (0.64°), λ = 1.54 Å and θ ≈ 18°, the average size of the Fe3O4 nanoparticles is estimated to be around 13 nm. Transmission electron microscopy (TEM) analyses were used for characterization (Fig. 3). The TEM image reveals the spherical Fe3O4 nanoparticles with the average size of 10–20 nm, consistent with average size obtained from the Debye–Scherrer formula. The FT-IR spectra of Fe3O4 nanoparticles are shown in Fig. 4. The absorbance bands at 584.3 cm−1 ascribed to Fe+2 O−2 are consistent with the reported IR spectra for spinel Fe3O4.29,30
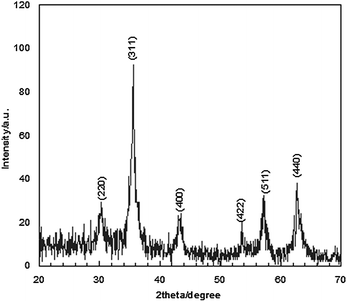 | ||
| Fig. 2 The powder X-ray diffraction pattern of the Fe3O4 nanoparticles. | ||
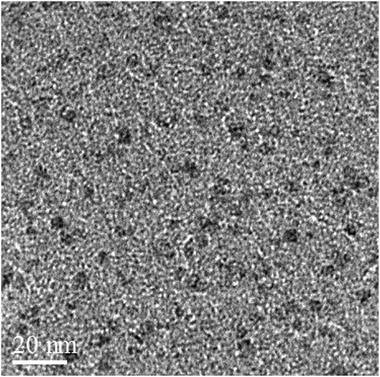 | ||
| Fig. 3 TEM image shows spherical Fe3O4 nanoparticles of 10–20 nm. | ||
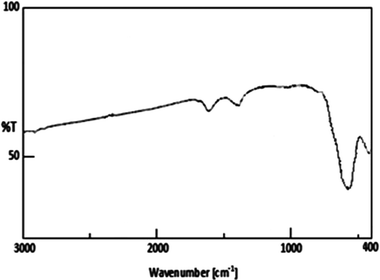 | ||
| Fig. 4 FT-IR spectra of Fe3O4 nanoparticles. | ||
Previously, xanthene and its derivatives have been synthesized under reflux for 4–5 h in dichloromethane or 1,2-dichloroethane as solvent in the presence of acid catalysts such as tetra-n-butylammonium fluoride.31 These synthetic methods afforded good yield, however, have limitations of long reaction times, harsh reaction conditions and often expensive catalysts.
Therefore in this work we report a simple, efficient and practical approach for the synthesis of xanthene derivatives in the presence of magnetic nanoparticles Fe3O4 as eco-friendly catalysts with high catalytic activity at 80 °C in water as a solvent (Scheme 1).
Under the given conditions several aromatic aldehydes 2 containing electron donating as well as electron withdrawing groups with different substitution patterns were effectively cyclized to give 9-aryl substituted 1,8-dioxo-octahydroxanthenes (Table 1). The product was isolated simply by filtration. Next, we examined the scope of the reaction by using various aromatic aldehydes, and the results are summarized in Table 1. In all the cases corresponding xanthene derivatives were obtained in good to excellent yields.
The Fe3O4 nanoparticle catalyst plays a crucial role in the success of the reaction. In the absence of the Fe3O4 nanoparticle catalyst, the reaction of 1,3-cyclohexanediones 1 and benzaldehyde derivatives 2 in the presence of the Fe3O4 powder as a catalyst could be carried out, but the product was obtained in very low yield after prolonged time (Table 3). Since metal oxides play the role as a heterogeneous catalyst in many chemical industries and the rate of reaction on the catalyst surface depends on the total surface area and the number of active sites on the catalyst, a good catalytic activity is obtained from smaller particle size and high surface area of the catalyst.32 As can be seen from Table 3, when the same weights of nanoparticle and powdered Fe3O4 were handled, the crucial role of Fe3O4 nanoparticles as a good catalyst was obviously revealed.
| Entry | Aldehyde | Product | Nanoparticles Fe3O4 | Fe3O4 powder |
|---|---|---|---|---|
| Time (min)/Yielda (%) | Time (min)/Yielda (%) | |||
| a Refers to isolated yields. | ||||
| 1 |

|

|
8/96 | 260/25 |
| 2 |

|

|
37/89 | 300/30 |
| 3 |

|

|
33/90 | 280/30 |
The nature of iron sites as Lewis acids causes the reactant with functional groups such as carbonyl (–CO), nitrile (–CN), hydroxyl (–OH), thiol (–SH) or sulfur dioxide (–SO2) and etc. to undergo chemical adsorption by interaction with the acidic surface of metal sites. In the aqueous phase, the water molecule gets associatively adsorbed with the Lewis basic oxygen lone pair orbital onto a Lewis acidic surface of the Fe site, then the substrate adsorbate bond gets established by interaction between the water HOMO orbital and empty Fe 3d orbitals that are energetically located in the lower conduction band region. Because of interaction between the carbonyl group of the substrate with Fe 3d orbitals of the catalyst, the carbonyl group of aldehyde was activated for nucleophilic attack in next steps and causes expedition of reaction progress.33–35
Comparison of this method with others for synthesis of 3,3,6,6-tetramethyl-9-phenyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8 (2H)-dione (Table 1, entry 1) as a model reaction is shown in Table 4.15,18,22,36–40
| Entry | Catalyst | mol% | Solvent/Temp. (°C) | Time (min) | Reference |
|---|---|---|---|---|---|
| a p-Toluenesulfonic acid. b p-Dodecylbenzenesulfonic acid. c Trimethylsilyl chloride. d Tetrabutylammonium hydrogen sulfate. e 1-(Chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2,2,2]octane bis(tetrafluoroborate). f Polyphosphoric acid supported on silica. | |||||
| 1 | Nanoparticle Fe3O4 | 1 | H2O/80 | 8 | This work |
| 2 | p-TsOHa | 5 | MeOH, H2O/80 | 20 | 36 |
| 3 | DBSAb | 10 | H2O-Ultrasonic/25–30 | 60 | 37 |
| 4 | TMSClc | 100 | CH3CN/Reflux | 420 | 38 |
| 5 | TBAHSd | 10 | Dioxane, H2O/Reflux | 210 | 22 |
| 6 | DBSAb | 20 | H2O/Reflux | 180 | 39 |
| 7 | SelectfluorTMe | 10 | Solvent free/120 | 60 | 40 |
| 8 | PPA-SiO2f | 10 | Solvent free/140 | 30 | 15 |
| 9 | HClO4-SiO2 | 10 | Solvent free/140 | 180 | 15 |
| 10 | SbCl3-SiO2 | 10 | Solvent free/120 | 50 | 18 |
These results show that this catalyst provided the best conditions for the synthesis of xanthene derivatives than other catalysts and methods that were reported. We also studied the effect of the amount of the catalyst on this reaction. We found that the best amount of the catalyst is 1 mol% for the model reaction whereas the larger amounts of the catalyst did not improve the results to a greater extent (Table 5).
| Product | mol% | Yield (%) | Time (min) |
|---|---|---|---|

|
1 | 96 | 8 |
| 5 | 96 | 8 | |
| 10 | 95 | 9 | |
| 20 | 94 | 9 |
Conclusions
In summary, a convenient and highly efficient method for the synthesis of 9-aryl substituted 1,8-dioxo-octahydroxanthenes by condensation reaction of aldehydes with 1,3-cyclohexanediones by the use of magnetic nanoparticles Fe3O4 as an inexpensive and eco-friendly catalyst has been described. The attractive features of this protocol are simple workup procedure, very short reaction time, and reusability of the catalyst. Furthermore, products were isolated in excellent yields. Also the catalyst was efficiently recovered and reused, which is considered as an economic advantage of this synthesis.Acknowledgements
Financial support from Yasouj University of Iran was greatly acknowledged.References
- A. M. El-Brashy, M. E. Metwally and F. A. El-Sepai, Farmaco, 2004, 59, 809–817 CrossRef CAS.
- K. Chibale, M. Visser, D. V. Schalkwyk, P. J. Smith, A. Saravanamuthu and A. H. Fairlamb, Tetrahedron, 2003, 59, 2289–2296 CrossRef CAS.
- B. B. Bhowmik and P. Ganguly, Spectrochim. Acta, Part A, 2005, 61, 1997–2003 CrossRef.
- R. M. Ion, D. Frackowiak and K. Wiktorowicz, Acta Biochim. Pol., 1998, 45, 833–845 CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–689 CAS.
- M. Ahmad, T. A. King, D. K. Ko, B. H. Cha and J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473–1476 CrossRef CAS.
- G. Saint-Ruf, H. T. Hieu and J. P. Poupelin, Naturwissenschaften, 1975, 62, 584–585 CrossRef CAS.
- J. Kinjo, H. Uemura, T. Nohara, M. Yamashita, N. Marubayashi and K. Yoshihira, Tetrahedron Lett., 1995, 36, 5599–5602 CAS.
- G. Casiraghi, G. Casnati and M. Cornia, Tetrahedron Lett., 1973, 14, 679–682 CrossRef.
- A. Bekaert, J. Andrieux and M. Plat, Tetrahedron Lett., 1992, 33, 2805–2806 CrossRef CAS.
- D. W. Knight and P. B. Little, J. Chem. Soc., Perkin Trans. 1, 2001, 1771–1777 RSC.
- C. W. Kuo and J.-M. Fang, Synth. Commun., 2001, 31, 877–892 CrossRef CAS.
- A. Jha and J. Beal, Tetrahedron Lett., 2004, 45, 8999–9001 CrossRef CAS.
- M. Seyyedhamzeh, P. Mirzaei and A. Bazgir, Dyes Pigm., 2008, 76, 836–839 CrossRef CAS.
- S. Kantevari, R. Bantu and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53–57 CrossRef CAS.
- K. Venkatesan, S. S. Pujari, R. J. Lahoti and K. V. Srinivasan, Ultrason. Sonochem., 2008, 15, 548–553 CrossRef CAS.
- X. Fan, X. Hu, X. Zhang and J. Wang, Can. J. Chem., 2005, 83, 16–20 CrossRef CAS.
- Z. H. Zhang and Y. Lui, Catal. Commun., 2008, 9, 1715–1719 CrossRef CAS.
- N. G. Kozlov and L. I. Basalaeva, Russ. J. Gen. Chem., 2005, 75, 617–621 CrossRef CAS.
- E. C. Horning and M. G. Horing, J. Org. Chem., 1946, 11, 95–99 CrossRef CAS.
- K. h. Niknam and M. Damya, J. Chin. Chem. Soc. (Taipei), 2009, 56, 659–665 CAS.
- H. N. Karade, M. Sathe and M. P. Kaushik, ARKIVOC, 2007, xiii, 252–258 Search PubMed.
- B. Das, P. Thirupathi, I. Mahender, V. S. Reddy and Y. K. Rao, J. Mol. Catal. A: Chem., 2006, 247, 233–239 CrossRef CAS.
- A. John, P. J. P. Yadav and S. Palaniappan, J. Mol. Catal. A: Chem., 2006, 248, 121–125 CrossRef CAS.
- H. R. Tavakoli, H. Zamani, M. H. Ghorbani and H. Etedali Habibabadi, J. Iran. Chem. Soc., 2009, 2, 118–126 Search PubMed.
- A. Sachar, R. L. Sharma, S. Kumar, D. Kaur and J. Singh, J. Heterocycl. Chem., 2006, 43, 1177–1181 CrossRef CAS.
- Y. S. Kang, S. Rishbud, J. F. Rabolt and P. Stroeve, Chem. Mater., 1996, 8, 2209–2211 CrossRef CAS.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS.
- Y. Liu, P. Liu, Z. Su, F. Li and F. Wen, Appl. Surf. Sci., 2008, 255, 2020–2025 CrossRef CAS.
- S. C. Wuang, K. G. Neoh, E.-T. Kang, D. W. Pack and D. E. Leckband, J. Mater. Chem., 2007, 17, 3354–3362 RSC.
- S. Gao, C. H. Tsai and C. F. Yao, Synlett, 2009, 949–954 CAS.
- R. L. Augustine, Heterogeneous catalysis for the synthetic chemist, Marcel Dekker Inc., New York, 1996 Search PubMed.
- W. Weiss and R. Schlogl, Top. Catal., 2000, 13, 75–90 CrossRef CAS.
- J. Baltrusaitis, D. M. Cwiertny and V. H. Grassian, Phys. Chem. Chem. Phys., 2007, 9, 5542–5554 RSC.
- F. Niu, L. Zhang, S.-Z. Luo and W.-G. Song, Chem. Commun., 2010, 46, 1109–1111 RSC.
- K. Venkatesan, S. S. Pujari, R. J. Lahoti and K. V. Srinivasan, Ultrason. Sonochem., 2008, 15, 548–553 CrossRef CAS.
- T.-S. Jin, J.-S. Zhang, A.-Q. Wang and T.-S. Li, Ultrason. Sonochem., 2006, 13, 220–224 CrossRef CAS.
- S. Kantevari, R. Bantu and L. Nagarapu, ARKIVOC, 2006, xvi, 136–148 Search PubMed.
- L. L. Bin, J. T. Shou, H. L. Sha, L. Meng, Q. Na and L. T. Shuang, E-J. Chem., 2006, 3, 117–121 Search PubMed.
- M. R. Poor Heravi, J. Iran. Chem. Soc., 2009, 6, 483–488 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00289a |
| This journal is © The Royal Society of Chemistry 2012 |
