Energy analysis of weak electron-donor–acceptor complexes and water clusters with the perturbation theory based on the locally projected molecular orbitals: charge-transfer and dispersion terms
Abstract
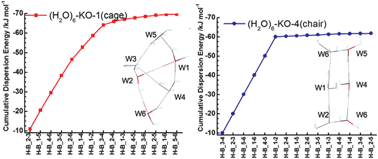
The third order single excitation perturbation theory corrected with the dispersion energy based on the locally projected molecular orbital was applied to study the weak electron-donor–acceptor (charge-transfer) complexes and the hydrogen bonds in the water clusters. In the weak electron-donor–acceptor complexes, the dispersion energy is larger than the charge-transfer energy in absolute value. The dispersion energy is as large as the charge-transfer energy in the hydrogen bond. The cage form of (H2O)6 is the most stable among eight isomers examined, because the dispersion energy is the largest among them.
- This article is part of the themed collection: Fragment and localized orbital methods in electronic structure theory

 Please wait while we load your content...
Please wait while we load your content...