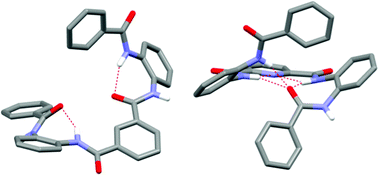
The crystal structures and molecular conformations of two foldamer-type oligoamides were analyzed. One polymorphic form and seven solvates were found for N1,N3-bis(2-benzamidophenyl)benzene-1,3-dicarboxamide (the benzene variant), and two polymorphic forms and six solvates for N2,N6-bis(2-benzamidophenyl)pyridine-2,6-dicarboxamide (the pyridine variant). Three crystal structures of the benzene variant and seven structures of the pyridine variant were solved using single crystal X-ray diffraction. The crystal structures showed that the different modes of intramolecular hydrogen bonding strongly affect the conformation and folding of the molecules, which is most evidently seen with the strongly folded helical structure of the pyridine variant. NOESY experiments suggest that the intramolecular hydrogen bonding is stable enough to retain a folded or partially folded conformation even in solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...