Enantioselective bromolactonization of cis-1,2-disubstituted olefinic acids using an amino-thiocarbamate catalyst†‡
Abstract
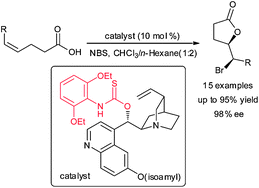
A facile, highly regio- and enantioselective amino-thiocarbamate-catalyzed bromolactonization of cis-1,2-disubstituted olefinic acids has been developed. The use of the enantio-enriched
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...