A Mn36Ni4 ‘loop-of-loops-and-supertetrahedra’ aggregate possessing a high ST = 26 ± 1 spin ground state†
Maria
Charalambous
a,
Eleni E.
Moushi
a,
Constantina
Papatriantafyllopoulou
ab,
Wolfgang
Wernsdorfer
c,
Vassilios
Nastopoulos
d,
George
Christou
b and
Anastasios J.
Tasiopoulos
*a
aDepartment of Chemistry, University of Cyprus, 1678 Nicosia, Cyprus. E-mail: atasio@ucy.ac.cy; Fax: +357 22892801; Tel: +357 22892765
bDepartment of Chemistry, University of Florida, Gainesville, FL 32611-7200, USA
cInstitut Néel, CNRS, BP-166, Grenoble Cedex 9, France
dDepartment of Chemistry, University of Patras, 26500 Patras, Greece
First published on 16th March 2012
Abstract
The initial use of 1,3-propanediol in mixed Mn/3d cluster chemistry has led to a MnIII28MnII8NiII4 molecular aggregate which consists of two MnIII8Ni2 loops and two MnIII6MnII4 supertetrahedral units and displays a high ground spin state value ST = 26 ± 1.
Polynuclear clusters of paramagnetic 3d metal ions have attracted intense interest in the last two decades for a number of reasons including their novel crystal structures and magnetic properties.1–3 One important challenge for coordination chemists is the utilization of polynuclear complexes with interesting magnetic properties for the construction of larger clusters or polymeric networks. Such compounds would combine novel structural features (large size, high symmetry, and aesthetically pleasing shapes and architectures) with retention and possibly enhancement of the magnetic properties of their ‘magnetic’ “building-blocks”. However, although there are a few coordination polymers composed of magnetically interesting Mn3,4 Mn4,5 Mn6,6 Mn10,7 Mn178 and Mn199 units, the list of discrete polynuclear complexes containing such “building-blocks” is very small, being limited mainly to some polynuclear clusters comprising linked trinuclear units.1b,10
We recently reported a family of large molecular aggregates consisting of four smaller clusters linked through Na+ or Mn2+ ions.11 These large tetrameric [Mn10M(μ3-O)2(O2CCH3)13(pd)6(py)2]4x+ (Mn40M4; pd = the dianion of 1,3-propanediol; M = Na+, x = 0; M = Mn2+, x = 1), clusters contain four Mn10 loops linked through Na+ or Mn2+ ions and have a saddle-like topology. The Mn44 analogue of this family displays a spin ST = 6 ground state and SMM behaviour. Further investigation of the reactions that afforded the Mn40M4 clusters involved the use of various 3d paramagnetic metal ions in an attempt to isolate a series of heterometallic Mn/3d analogues and/or other large aggregates composed of smaller clusters.
We herein report the initial result of these studies, which is the new molecular aggregate [MnIII28MnII8Ni4O12Cl10(O2CCH3)26(pd)24(py)4(H2O)2] (1) that possesses an unprecedented ‘loop-of-loops-and-supertetrahedra’ structural topology. It consists of two MnIII8Ni2 loops, which are related to the Mn10 loops of the Mn40M4 complexes, and two MnIII6MnII4 units exhibiting a supertetrahedral structural motif. The latter has appeared in several MnIII6MnII4 discrete complexes12 and is known to display intracluster ferromagnetic exchange interactions and a high ST = 22 ground state. The large MnIII28MnII8NiII4 cluster thus represents an unusual example of a molecular aggregate consisting of magnetically interesting polynuclear Mx (x > 6) repeating units. In addition, it possesses a high spin ground state ST = 26 ± 1, the highest yet observed for a mixed metal cluster and one of the highest for any metal cluster.
The reaction of [Mn3O(O2CMe)6(py)3]·py (py = pyridine) with H2pd and NiCl2·6H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in CH3CN resulted in a dark-brown slurry, which was filtered to give a brown filtrate. The filtrate was left undisturbed at room temperature for a few days, and it slowly gave red-brown crystals of 1·2CH3CN·12.30H2O in 35% yield; dried solid was analyzed as 1·10H2O.‡ The molecular structure of‡1 (Fig 1, top) consists of two mixed-metal [MnIII8Ni2(μ3-O)2(O2CCH3)12(pd)6(py)2] loops (Fig. 1, bottom, right) and two [MnIII6MnII4(μ4-Ο)4(μ3-Cl)4(O2CCH3)Cl(pd)6(H2O)] supertetrahedral units (Fig 1, bottom, left). The MnIII8Ni2 loops are related to the Mn10 loops of the Mn40M4 clusters, with the main difference being the existence of the two Ni2+ ions in the former instead of two Mn2+ ions. Thus, each MnIII8Ni2 unit consists of two [MnIII3O]7+ triangles and two dinuclear MnIIINiII subunits linked by pd2− μ-O atoms, and bridging CH3CO2− groups. The peripheral ligation of the MnIII8Ni2 loop is provided by six syn, syn-η1:η1:μ2, four η1:η2:μ3 and two η2:η2:μ4 CH3CO2− groups, six η2:η2:μ3 pd2− ligands (the coordination modes of pd2− ligand in 1 is illustrated in Fig. S1, in ESI†) and two terminal py molecules. Two μ3 and one μ CH3CO2− ligands connect the Mn ions of each [MnIII3O]7+ triangle of the MnIII8Ni2 loops to a MnII ion of a MnIII6MnII4 supertetrahedral unit (Fig. S2, in ESI†) resulting in the formation of the nearly-planar MnIII28MnII8Ni4 ‘loop-of-loops-and-supertetrahedra’ aggregate. The [MnIII6MnII4(μ4-Ο)4(μ3-Cl)4(O2CCH3)Cl(pd)6(H2O)] subunit (Fig 1, bottom, left) consists of a [MnIII6MnII4(μ4-Ο)4]18+ supertetrahedral core which is analogous to those observed in other discrete Mn10 complexes reported recently.12 Each Mn10 supertetrahedron contains nine Mn ions in two stacked Mn3 and Mn6 isosceles triangles, and a tenth Mn at the apex position. Its Mn6 base consists of three MnII and three MnIII atoms located at the corners and the edges of an isosceles triangle, respectively. The Mn10 unit is held together by four μ4-Ο2− ligands resulting in a [MnII4MnIII6(μ4-Ο)4]18+ core (Fig. S3 in ESI†). The peripheral ligation of the supertetrahedron is completed by one syn,syn-η1:η1:μ2 CH3CO2− group, six η2:η2:μ3 pd2− ligands, four μ3 and one terminal Cl− ions and one monodentate H2O molecule. The oxidation states of the Mn ions and the protonation levels of O2−/RO−/RCO2− groups were determined by bond valence sum (BVS) calculations,13 charge balance considerations, and inspection of metric parameters.
1 molar ratio in CH3CN resulted in a dark-brown slurry, which was filtered to give a brown filtrate. The filtrate was left undisturbed at room temperature for a few days, and it slowly gave red-brown crystals of 1·2CH3CN·12.30H2O in 35% yield; dried solid was analyzed as 1·10H2O.‡ The molecular structure of‡1 (Fig 1, top) consists of two mixed-metal [MnIII8Ni2(μ3-O)2(O2CCH3)12(pd)6(py)2] loops (Fig. 1, bottom, right) and two [MnIII6MnII4(μ4-Ο)4(μ3-Cl)4(O2CCH3)Cl(pd)6(H2O)] supertetrahedral units (Fig 1, bottom, left). The MnIII8Ni2 loops are related to the Mn10 loops of the Mn40M4 clusters, with the main difference being the existence of the two Ni2+ ions in the former instead of two Mn2+ ions. Thus, each MnIII8Ni2 unit consists of two [MnIII3O]7+ triangles and two dinuclear MnIIINiII subunits linked by pd2− μ-O atoms, and bridging CH3CO2− groups. The peripheral ligation of the MnIII8Ni2 loop is provided by six syn, syn-η1:η1:μ2, four η1:η2:μ3 and two η2:η2:μ4 CH3CO2− groups, six η2:η2:μ3 pd2− ligands (the coordination modes of pd2− ligand in 1 is illustrated in Fig. S1, in ESI†) and two terminal py molecules. Two μ3 and one μ CH3CO2− ligands connect the Mn ions of each [MnIII3O]7+ triangle of the MnIII8Ni2 loops to a MnII ion of a MnIII6MnII4 supertetrahedral unit (Fig. S2, in ESI†) resulting in the formation of the nearly-planar MnIII28MnII8Ni4 ‘loop-of-loops-and-supertetrahedra’ aggregate. The [MnIII6MnII4(μ4-Ο)4(μ3-Cl)4(O2CCH3)Cl(pd)6(H2O)] subunit (Fig 1, bottom, left) consists of a [MnIII6MnII4(μ4-Ο)4]18+ supertetrahedral core which is analogous to those observed in other discrete Mn10 complexes reported recently.12 Each Mn10 supertetrahedron contains nine Mn ions in two stacked Mn3 and Mn6 isosceles triangles, and a tenth Mn at the apex position. Its Mn6 base consists of three MnII and three MnIII atoms located at the corners and the edges of an isosceles triangle, respectively. The Mn10 unit is held together by four μ4-Ο2− ligands resulting in a [MnII4MnIII6(μ4-Ο)4]18+ core (Fig. S3 in ESI†). The peripheral ligation of the supertetrahedron is completed by one syn,syn-η1:η1:μ2 CH3CO2− group, six η2:η2:μ3 pd2− ligands, four μ3 and one terminal Cl− ions and one monodentate H2O molecule. The oxidation states of the Mn ions and the protonation levels of O2−/RO−/RCO2− groups were determined by bond valence sum (BVS) calculations,13 charge balance considerations, and inspection of metric parameters.
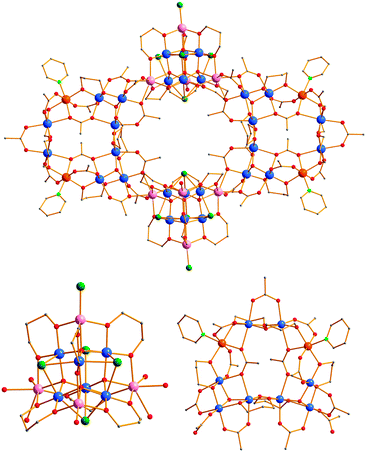 | ||
| Fig. 1 Representations of the molecular structure of 1 (top) and its MnIII6MnII4 supertetrahedral (bottom, left) and MnIII8Ni2 loop (bottom, right) subunits. Colour code: MnIII, blue; MnII, lavender; NiII, orange; O, red; N, light green; Cl, green; C, gray. H atoms are omitted. | ||
Compound 1 possesses an aesthetically pleasing topology and displays an unusually large nuclearity and size, being one of the largest heterometallic MnxMy (M = any metal ion) metal clusters.14 It is also interesting that the Mn36Ni4 cluster consists of two high nuclearity complexes which display structural cores that have appeared in the past in discrete complexes and/or in fragments of larger clusters. In particular, the [MnII4MnIII6(μ4-O4)]18+ supertetrahedral core has appeared in several MnIII6MnII4 discrete complexes12 and also has been recognized as a fragment in larger, Mn178a,15 and Mn19 clusters.2 In all cases, intracluster ferromagnetic exchange interactions were realized, which resulted in high ST = 22 or abnormally high ST = 37 and 83/2 spin ground state values for the discrete MnIII6MnII4 and the Mn17 and Mn19 complexes, respectively. For these reasons, the MnIII6MnII4 supertetrahedral unit represents a very attractive “building-block” for the construction of large clusters and multidimensional coordination polymers.7 Thus, the presence in 1 of the MnIII6MnII4 unit is expected to result in dominant intracluster ferromagnetic exchange interactions and a very high spin ground state.
Solid-state, direct-current (dc) magnetic susceptibility (χM) data were collected in the 5–300 K range in a 1 kG (0.1 T) field and are plotted as χMT vs. T in Fig. 2. The χΜT value at 300 K is 118.63 cm3 mol−1 K and increases steadily with decreasing temperature to 325.63 cm3 mol−1 K at 15 K, and then decreases to 304.34 cm3 mol−1 K at 5.0 K. This behaviour is indicative of the existence of dominant ferromagnetic exchange interactions in 1. In addition, the maximum of 325.63 cm3 mol−1 K at 15 K is consistent with an S in the 25 to 27 range, depending on the g value. The small decrease at the lowest temperatures is assigned to Zeeman effects, zero-field splitting and/or weak intermolecular interactions. To determine the ground state of 1, magnetisation (M) data were collected in the 1–10 kG and 1.8–4.0 K ranges, and these are plotted as reduced magnetisation (M/NμB) vs. H/T in Fig. S4 (ESI†). The data were fit by assuming that only the ground state is populated and by including axial zero-field splitting (DŜ2z) and isotropic Zeeman interactions. Equal quality fits were obtained for S = 25, 26, and 27 with parameters g = 2.03(1)/D = −0.007(1) cm−1, g = 1.96(1)/D = −0.004(1) cm−1, and g = 1.91(1)/D = −0.004(1) cm−1, respectively. We conclude that 1 has a ground state of ST = 26 ± 1, and a very small D value.
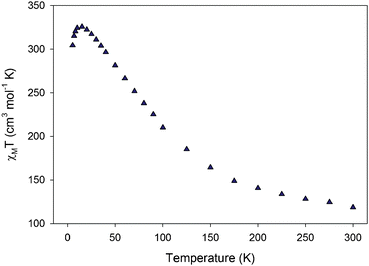 | ||
| Fig. 2 Plot of χMT vs. T for complex 1. | ||
Confirmation of the ground state values proposed for 1 on the basis of the dc studies was obtained by alternating current (ac) susceptibility experiments. Ac susceptibility studies use no dc field and thus are an excellent complementary tool for determining S by avoiding potential complications from a large dc field.3a,9,11,12a The in-phase susceptibility 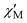 for 1 is shown as
for 1 is shown as 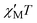 versus T in Fig. 3, and extrapolation of the
versus T in Fig. 3, and extrapolation of the 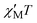 signal to 0 K from above ∼8 K (to avoid the effects of intermolecular interactions at lower temperatures) gives a value of ∼340 cm3 mol−1 K consistent with: (i) S = 25 and g = 2.05, (ii) S = 26 and g = 1.97, and (iii) S = 27 and g = 1.90. The AC data thus confirm that 1 possesses a high ground state spin value of ST = 26 ± 1. Examination of the out-of-phase ac plot
signal to 0 K from above ∼8 K (to avoid the effects of intermolecular interactions at lower temperatures) gives a value of ∼340 cm3 mol−1 K consistent with: (i) S = 25 and g = 2.05, (ii) S = 26 and g = 1.97, and (iii) S = 27 and g = 1.90. The AC data thus confirm that 1 possesses a high ground state spin value of ST = 26 ± 1. Examination of the out-of-phase ac plot 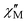 vs. T (Fig. S5 in ESI†) reveals that complex 1 does not exhibit an out-of-phase ac magnetic susceptibility signal down to 1.8 K.
vs. T (Fig. S5 in ESI†) reveals that complex 1 does not exhibit an out-of-phase ac magnetic susceptibility signal down to 1.8 K.
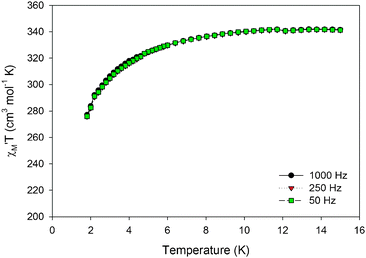 | ||
Fig. 3 Plot of the in-phase (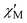 ) (as ) (as 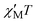 ) ac magnetic susceptibility versus T for complex 1 at the indicated frequencies. ) ac magnetic susceptibility versus T for complex 1 at the indicated frequencies. | ||
To confirm whether 1 is a SMM, magnetisation versus dc field scans were carried out on a single crystal of 1·2CH3CN·12.30H2O using a micro-SQUID apparatus.16 These studies revealed the existence of hysteresis loops below 0.3 K but with a very narrow coercivity (Fig. S6 in ESI†) that increased slightly with either decreasing temperature or increasing scan rate. This is not a typical SMM behaviour for which one would normally expect wider loops and a greater dependence of the coercivity on the temperature. We thus conclude that 1 is not a SMM. This could be attributed to its very small D value as determined by variable field—variable temperature magnetisation measurements. Such a small D value for 1 is not surprising since the highly symmetric MnIII6MnII4 supertetrahedral unit that it contains is known to display a D ≈ 0 and as a consequence not to be a SMM. Clearly there are remarkable analogies between the overall magnetic behaviour of 1 and its MnIII6MnII4 supertetrahedral “building-block” as both clusters display ferromagnetic exchange interactions, high spin ground state, a nearly zero D, and do not exhibit SMM behaviour.12
In summary, the initial employment of H2pd in mixed metal chemistry has led to a large MnIII28MnII8NiII4 cluster which consists of two MnIII8Ni2 loops and two MnIII6MnII4 supertetrahedral units. It represents a relatively rare example of a large cluster consisting of covalently linked polynuclear Mx (x > 6) complexes and the only one that contains a magnetically interesting polynuclear Mx repeating unit. The presence of the ST = 22 MnIII6MnII4 supertetrahedral unit in the structure of 1 affected dramatically the overall magnetic behaviour of the latter resulting in a high ST = 26 ± 1 spin ground state, the highest observed in a heterometallic cluster. The isolation of 1 suggests that other aggregates that will contain only MnIII6MnII4 or other magnetically interesting high nuclearity complexes as “building-blocks” are also possible. Such complexes could be of significant interest not only for their aesthetically pleasing structures but also for their magnetic properties since for example a cluster consisting of only MnIII6MnII4 supertetrahedral units would likely have a giant ground spin state. Thus, further investigations targeting to the isolation of a series of analogues of 1 and/or large clusters composed of magnetically interesting MnxMy complexes are in progress.
This work was supported by the Cyprus Research Promotion Foundation Grant “ANABAΘMIΣH/ΠAΓIO/0308/12” which is co-funded by the Republic of Cyprus and the European Regional Development Fund and the USA NSF (CHE-0910472).
Notes and references
- (a) G. Aromi and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef CAS; (b) E. K. Brechin, Chem. Commun., 2005, 5141 RSC; (c) A. J. Tasiopoulos and S. P. Perlepes, Dalton Trans., 2008, 5537 RSC; (d) G. Christou, Polyhedron, 2005, 24, 2065 CrossRef CAS.
- A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 4926 CrossRef CAS.
- (a) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC; (b) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS.
- (a) H.-B. Xu, B.-W. Wang, F. Pan, Z.-M. Wang and S. Gao, Angew. Chem., Int. Ed., 2007, 46, 7388 CrossRef CAS; (b) Y.-L. Bai, J. Tao, W. Wernsdorfer, O. Sato, R.-B. Huang and L.-S. Zheng, J. Am. Chem. Soc., 2006, 128, 16428 CrossRef CAS.
- (a) L. Lecren, O. Roubeau, C. Coulon, Y.-G. Li, X. F. Le Goff, W. Wernsdorfer, H. Miyasaka and R. Clérac, J. Am. Chem. Soc., 2005, 127, 17353 CrossRef CAS; (b) H. Miyasaka, K. Nakata, L. Lecren, C. Coulon, Y. Nakazawa, T. Fujisaki, K. Sugiura, M. Yamashita and R. Clérac, J. Am. Chem. Soc., 2006, 128, 3770 CrossRef CAS.
- (a) L. F. Jones, A. Prescimone, M. Evangelisti and E. K. Brechin, Chem. Commun., 2009, 2023 RSC; (b) A. D. Katsenis, R. Inglis, A. Prescimone, E. K. Brechin and G. S. Papaefstathiou, CrystEngComm, 2012, 14, 1216 RSC.
- G. Wu, J. Huang, L. Sun, J. Bai, G. Li, E. Cremades, E. Ruiz, R. Clérac and S. Qiu, Inorg. Chem., 2011, 50, 8580 CAS.
- (a) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Inorg. Chem., 2009, 48, 5049 CrossRef CAS; (b) E. E. Moushi, T. C. Stamatatos, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Polyhedron, 2009, 28, 1814 CrossRef CAS.
- E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2006, 45, 7722 CrossRef CAS.
- (a) R. Inglis, C. J. Milios, L. F. Jones, S. Piligkos and E. K. Brechin, Chem. Commun., 2012, 48, 181 RSC; (b) T. N. Nguyen, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2011, 133, 20688 CrossRef CAS; (c) B. Cordero, O. Roubeau, S. J. Teat and A. Escuer, Dalton Trans., 2011, 40, 7127 RSC.
- (a) E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Inorg. Chem., 2007, 46, 3795 CrossRef CAS; (b) E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146 CrossRef CAS.
- (a) T. C. Stamatatos, K. A. Abboud, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2006, 45, 4134 CrossRef CAS; (b) M. Manoli, R. D. L. Johnstone, S. Parsons, M. Murrie, M. Affronte, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2007, 46, 4456 CrossRef CAS; (c) S. Nayak, M. Evangelisti, A. K. Powell and J. Reedijk, Chem.–Eur. J., 2010, 16, 12865 CrossRef CAS.
- (a) W. Liu and H.-H. Thorp, Inorg. Chem., 1993, 32, 4102 CrossRef CAS; (b) I. D Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 244 CrossRef.
- W.-G. Wang, A.-J. Zhou, W.-X. Zhang, M.-L. Tong, X.-M. Chen, M. Nakano, C. C. Beedle and D. N. Hendrickson, J. Am. Chem. Soc., 2007, 129, 1014 CrossRef CAS.
- S. Nayak, L. M. C. Beltran, Y. Lan, R. Clérac, N. G. R Hearns, W. Wernsdorfer, C. E. Anson and A. K. Powell, Dalton Trans., 2009, 1901 RSC.
- W. Wernsdorfer, Adv. Chem. Phys., 2001, 118, 99 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Various structural and magnetism figures. CCDC 862029 (1·2CH3CN·12.30H2O). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cc30654a |
‡ Vacuum-dried solid analyzed (C, H, N) as 1·10H2O. Calcd. (found): C, 26.19 (26.32); H, 4.06 (4.09); N, 0.85 (0.94)%. Metal analysis was performed via ICP-OES. Calcd. for 1·10H2O (found): Mn, 29.94 (30.09); Ni, 3.55 (3.68)%. Crystal data for 1·2CH3CN·12.30H2O: C148H276.60Cl10Mn36N6O126.30Ni4, M = 6728.33, monoclinic, a = 47.463(2) Å, b = 14.049(1) Å, c = 50.160 (2) Å, β = 103.689(3)°, V = 32496(2) Å3, T = 100(2) K, space group I2/a, Z = 4, ρcalcd = 1.375 g cm−3, 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 reflections collected, 11 529 reflections collected, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 reflections used, R1 [I > 2σ(I)] = 0.0725, wR2 = 0.1702. The asymmetric unit also contains severely disordered water molecules that could not be modeled properly. Thus, the SQUEEZE program was used to eliminate the contribution of the electron density in the disordered solvent region from the overall intensity data. 651 reflections used, R1 [I > 2σ(I)] = 0.0725, wR2 = 0.1702. The asymmetric unit also contains severely disordered water molecules that could not be modeled properly. Thus, the SQUEEZE program was used to eliminate the contribution of the electron density in the disordered solvent region from the overall intensity data. |
| This journal is © The Royal Society of Chemistry 2012 |
