Progress on the analytical methodology for biological volatile organic compounds
Zhuomin
Zhang
*,
Yunjian
Ma
and
Gongke
Li
*
School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China. E-mail: cesgkl@mail.sysu.edu.cn; zzm@mail.sysu.edu.cn; Fax: +86-20-84115107; Tel: +86-20-84110922
First published on 1st November 2012
Abstract
Biological terminal metabolites are volatile organic compounds (VOCs) with strong volatility, containing important bio-information related with biological metabolism. As a crucial research precondition, analytical methodology for biological VOCs has attracted much attention to achieve the adequate composition information of biological VOCs at different physiological status and metabolism phases. In this article, the analytical methodology for biological VOCs is summarized, focusing on the latest advances on sampling and analytical techniques followed by the preliminary application of distilling potential, but crucial, bio-information. Systematic study of the relationship among biological VOCs, biological VOC characteristics and corresponding biomarkers would result in a potential but promising research field, bioodoromics. The concept of bioodoromics is described, and the developing trend of bioodoromics in the future is proposed in this paper. It is believed that bioodoromics possesses the power to describe potential but crucial bio-information related with metabolism pathway. The study of biological VOCs and potential bioodoromics would facilitate relevant fields such as insect prevention, disease diagnosis, criminal track-down, agricultural product quality control and food safety, etc.
1. Introduction
Scientists found that female anopheles mosquitoes produce a sharp reaction to 4-methylphenol from human sweat. The odor directs anopheles mosquitoes to bite a person's skin precisely.1 Behavior ecologists find that through the smell many organisms can identify genetic relationships to some extent.2 In our daily life, there are so many examples related with impalpable or intangible “bioodor”. The substance base of bioodor is all kinds of biological volatile organic compounds (VOCs).Biological terminal metabolites are VOCs with strong volatility, containing important bio-information related with biological metabolism. Species and amounts of biological VOCs of every biological sample are unique and stable with “fingerprint” characteristics, namely biological VOC characteristics. The variation of species and amounts of biological VOC characteristics at different physiological status or metabolism processes contains important biomarkers and corresponding bio-information.3 Interpreting the relationship among biological VOCs, biological VOC characteristics and corresponding biomarkers would result in a potential but promising scientific field, bioodoromics. Similar to genomics and proteomics, bioodoromics possesses the power to describe potential but crucial bio-information related to metabolism pathway. The study of bioodoromics would facilitate relevant fields such as insect prevention, disease diagnosis, criminal track-down, agricultural product quality control and food safety, etc..
The development of gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS) technique in the early 1990s, spurred the rapid development of the study of biological VOCs, and a large number of papers have been published in this field until today. However, due to the lack of highly selective sampling technique and sensitive detection technique with high throughput for trace biological VOCs from complicated biological samples, until today the study of bioodoromics progresses at a slow pace and still remains at the level of primary qualitative and quantitative analysis of biological VOC composition.
The research framework from biological VOCs to bioodoromics has been summarized in Fig. 1. The main research goals of the new born bioodoromics lie in the qualification and quantification of entire biological VOC components, monitoring changes of biological VOC characteristics and distilling potential bio-information related to metabolic processes. Lack of effective analytical methodology for biological VOCs would cause the loss of biological VOC components and further important bio-information. Thus, how to improve sampling completeness and avoid bio-information loss has been one of main bottle-necks in this field. Development of effective analytical methodology for biological VOCs including the efficient sampling techniques with high extraction capacity and selectivity and sensitive analytical method with high throughput would facilitate the potential but rapidly growing bioodoromics.
 | ||
| Fig. 1 Schematic and critical diagram summarizing the research framework from biological VOCs to bioodoromics. | ||
2. Sampling technique
Since biological VOC composition is complex with significantly different structures and polarity, and the amounts of biological VOCs are usually at trace level from complicated biological samples. Thus, it is a tough task to sample biological VOCs entirely without efficient sampling techniques. Efficient sampling techniques for biological VOCs should possess a wide sampling range, high extraction capacity and selectivity, and can be conveniently coupled with sequent analytical instruments. The key to develop novel efficient and nondestructive sampling techniques for trace biological VOCs lies in the development of new enrichment media with higher extraction capacity and selectivity and establishment of new sampling techniques based on new enrichment media.2.1 Enrichment medium
Biological VOCs mainly include alkanes, alkenes, alcohols, esters, aromatic compounds, etc. Novel enrichment media for biological VOCs should possess excellent adsorption property with high extraction capacity and selectivity. Nowadays, synthesis of novel gas adsorption material is one of the hotspots in material science, and many novel gas adsorption materials such as nanometer-scale gas sensing material, nanoarray gas adsorption material, composite gas adsorption material and metal-organic frameworks (MOFs) material have been proposed as potentially effective enrichment media for biological VOCs.Nanometer-scale gas sensing material has received steadily growing attention for the VOC detection due to its peculiar and fascinating gas adsorptive property due to their small particle size and large surface area. Some nanometal oxides such as SnO2,4 Mn3O45 and WO36 and polymer nanoparticles such as polypyrrole nanoparticles7 demonstrate the excellent gas adsorptive capacity and have been widely used as gas sensing materials for VOC detection. Kida et al.4 developed a new gas sensor based on monodispersed SnO2 nanocrystals which exhibited high sensor responses to volatile ethanol, formaldehyde and toluene at 5–200 μg mL−1. Wang et al.6 developed a 10 atom% Cr-doped WO3 based chemi-resistive sensor which showed a fast, stable, fairly sensitive and highly selective response to low concentrations of acetone (0.2–1 μg mL−1). It is expected to be a good candidate for diabetes diagnosis based on human breath analysis.6 Compared with common nanometer-scale gas sensing material, nanoarray gas absorption material possesses the larger surface area and extraction capacity for VOCs, since its regular nanoarray structure can improve material thickness and density. Regular nanoarray structural material with the proper interspace could usually offer a larger surface area than randomly oriented material and was suitable for the adsorption of proper-sized gas molecules. Some novel nanoarray gas adsorption materials such as one-dimensional ZnO nanorod8 and nanoporous array anodic alumina (NAAA)9 have been prepared as extraction media for sampling biological VOCs. Coupled with suitable detection technique, the excellent analytical sensitivity can be achieved from ng to pg level. Zhang et al.9 proposed a sensitive, reliable and convenient analytical method for the potential study of trace biological VOCs of Bailan flower, stinkbug and orange peel samples by novel NAAA solid phase microextraction (SPME) fiber coating coupled with GC/MS.
Apart from extraction capacity, extraction selectivity is another crucial property of enrichment media. Nowadays, some composite gas adsorption materials have been proposed as extraction media of VOCs. Composite gas adsorption materials are usually prepared based on pure metal oxide gas sensing materials doping with polymer compounds such as ZnO/PDMS,10 (PPy)x/MoO3,11 carbon nanotube/hexa-peri-hexabenzocoronene (CNT/HBC-C12) bilayers12 or metal oxides such as ZnO/TiO2 (ref. 13) and hybrid CuxO/TiO2.14 These composite materials can introduce corresponding functional groups or arouse better size effect, which would facilitate the improvement of extraction selectivity for biological VOCs. Haick et al.12 produced a novel gas sensing material of CNT/HBC-C12 bilayers and formed a new sensor array. The new sensor array had good discriminative abilities to detect and distinguish different nonpolar VOC biomarkers of cancer. MOFs represent a new class of porous materials that can offer unmatched advantages for the selective enrichment of biological VOCs due to their ordered structure, high thermal stability, adjustable chemical functionality, ultra-high porosity and the availability of hundreds of crystalline and well-characterized porous structure.15,16 Yan group15 has conducted a lot of research on the development of new VOC sampling techniques by use of MOFs as extraction media. They used MOF-5 as sorbent for in-field sampling formaldehyde coupled with thermal desorption (TD)-GC/MS.17 Also, they prepared MOF-based tandem molecular sieves as a dual platform for selective microextraction and high-resolution gas chromatographic separation of n-alkanes in complex matrixes including human serum.18 The proposed tandem molecular sieves offered good enhancement factors from 235 for hexane to 1212 for nonane with wide linearity. Their research shows that MOFs have good potential as extraction media for biological VOCs.
2.2 Sampling technique
After the synthesis of novel enrichment media, the related sampling techniques for biological VOCs should be developed. The traditional sampling techniques for biological VOCs are mainly liquid–liquid extraction (LLE),19 steam distillation (SD),20 simultaneous distillation extraction (SDE),21 supercritical fluid extraction (SFE),22 and purge-and-trap (P&T).23 SD and SDE always require long extraction time, large amounts of solvents and multiple steps with the possibility of thermal decomposition of biological VOCs. SFE shows high selectivity to biological VOC representative components. However, the lack of suitable solvents for polar biological VOCs and the high expense for SFE analysis still limit its real application in this field. P&T possesses high extraction capacity and selectivity for different biological VOCs and can achieve easy connection to the consequent GC or GC/MS.24–26 Although P&T has been primarily used for the off-line sampling of biological VOCs, large-scale instruments for P&T and thermal desorption-colded injection procedure during P&T processes make this technique unsuitable for the on-field and in vivo sampling biological VOCs.Until now, microextraction techniques are simple, miniaturized, rapid and environmentally-friendly, and they represent a major part of modern sample preparation techniques for biological VOCs. Especially, microextraction technique can be used as a non-invasive sampling method for in vivo sampling a very small volume of headspace extraction phase. These features allow microextraction technique for in vivo sampling and monitoring of trace biological VOCs from complicated biological samples.27 SPME,28,29 stir bar sorptive extraction (SBSE),30,31 solid phase microextraction membrane (SPMEM)32–34 and needle trap (NT)35,36 as the branches of microextraction technique have been successfully used for the real sampling projects of biological VOCs. Headspace microextraction techniques are most frequently used for sampling biological VOCs from plants, animals, microorganisms, etc.27
Although current commercial microextraction techniques have been primarily applied for sampling biological VOCs, they still possess some limitations and restrict their application. Lack of specialized microextraction media is one of the obvious limitations, which hinders the improvement of extraction capacity and selectivity. Nowadays, some novel extraction media mentioned above have been successfully fabricated and prepared as novel microextraction techniques for sampling biological VOCs such as one-dimensional nanoarray9 and molecularly imprinted polymers (MIPs)37 SPME fiber coatings which usually demonstrate higher extraction capacity and selectivity to trace biological VOCs than commercial ones. As the derivative techniques of SPME, SBSE and SPMEM have greatly increasing amounts of extraction phase and higher surface area.30,33 Extraction phase volume of SBSE is usually 50–250 times larger than that of SPME fiber, which would lead to the further and dramatic improvement of extraction capacity. Meanwhile, there are many choices of suitable substrate materials for SBSE, SPMEM such as capillary glass tube, stainless steel wire, Pt wire, which facilitates the fabrication of novel extraction media on the surface of corresponding substrates and improvement of mechanical stability. NT is a potentially solventless sampling technique with introduction device. NT represents a novel and robust means of sample preparation for trace analysis in gaseous matrices and is quite suitable for rapid on-site sampling VOCs. After sampling the NT device can be easily transferred to the lab, and analytes can be conveniently desorbed in an inlet of analytical instrumentation and introduced for the further analysis. Moreover, NT devices packed with more than one adsorbent material represent a promising alternative to SPME for analysis of VOCs at the ng-pg level.38,39 Some physical or chemical fabrication methods such as physical coating, sol–gel, molecular imprinting, electroplating and chemical polymerization technique have been successfully applied to immobilize novel extraction media on substrates and result in various microextraction techniques. Moreover, SBSE, SPMEM and NT can avoid the cross contamination and be conveniently coupled with consequent analytical instruments such as GC/MS for the on-line analysis of biological VOCs. Penn et al.40 firstly studied the individual and gender fingerprints of human axillary odor using SBSE coupled with TD-GC/MS. The study provided useful help for the design of electronic sensors for biometric fingerprint and disease diagnoses. SPMEM technique has also been successfully used for the study of biological VOCs from human skin. Riazanskaia et al.41 developed a thermally-desorbed polydimethylsilicone membrane approach for in vivo sampling of VOCs in and on human skin coupled with GC/MS analysis. This method was expected to be used to record the VOC profiles present in skin diseases such as skin cancer. Mieth et al.42 used automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/time of flight (TOF)-MS for breath gas analysis in the clinical environment. The result suggested that needle trap combined with hyphenated chromatographic techniques optimally met the requirements of quantitative high-throughput analysis of volatile analytes in the clinical environment and worked well even in the presence of high concentrations of contaminants. Alonso et al.43 developed a headspace needle-trap method for the analysis of VOCs in human whole blood samples. A significant matrix effect can be eliminated by the simple dilution of the blood samples. The results suggest that headspace needle-trap technique is a good alternative to SPME methods for VOC analysis in complex biological matrices. Nowadays, apart from a few commercial SBSE and SPMEM coatings, there is a lack of novel SBSE and SPMEM coatings prepared for selectively sampling the biological VOCs, which has been a bottleneck for expanding their application range in the study of bioodoromics.
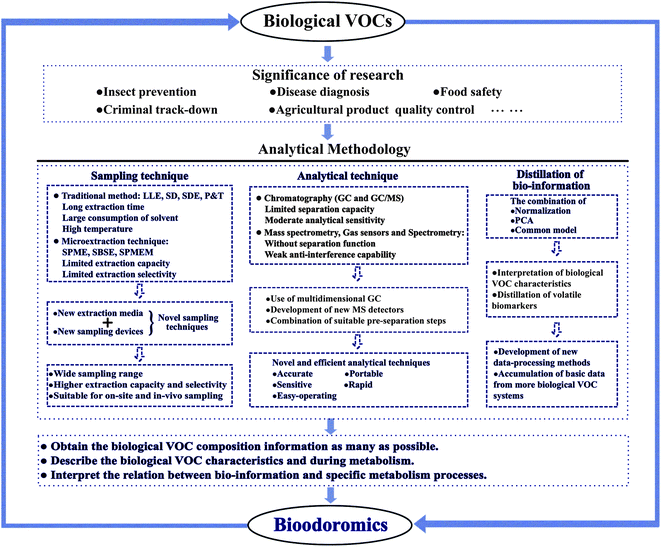
On the other hand, it is a crucial trend for on-site and in vivo sampling biological VOCs during real sampling projects by microextraction techniques coupled with suitable sampling devices. Typical sampling devices for biological VOCs should usually require a portable size, higher sample throughput and ease of use to prevent the sampling contamination. Nowadays, several sampling devices coupled with SPME have been developed and successfully applied for sampling the emanations from the human body,44,45 bovine breath,46 plant VOCs,47etc. Zhang et al.44 applied SPME to sample the emanations from human arm skin coupled with original active sampling systems and studied the emanation seasonal fingerprint characteristics coupled with GC/MS. Martin et al.45 conducted an in-situ sampling of salivary VOCs directly from the human oral cavity using a polydimethylsiloxane coupon. Compared with passive drool saliva collection, this new sampling method achieved a higher enhancement with reduced variability. Spinhirne et al.46 developed a relatively noninvasive sampling device consisting of a face mask sampling device and multi-SPME fibers for the identification of VOCs in bovine breath. Their results suggested that noninvasive heath screening using breath analyses could be a potential diagnostic tool for animal's diseases. Schwoebel et al.48 set up a breath sampling device for the analysis of phase-resolved real-time breath during exercise (Fig. 2). By use of this sampling device, continuous on-line breath gas sampling by proton-transfer reaction-linear ion trap mass spectrometer (PTR/MS) and alveolar off-line breath gas sampling in Tedlar® bags for SPME-GC/MS analyses can be achieved in parallel. However, we note that most sampling devices proposed for biological VOCs are designed coupled with SPME, and few devices are coupled with other sampling techniques. It is believable that more portable and efficient sampling devices which can be easily coupled with novel sampling techniques should be designed and proposed for the on-site and in vivo sampling original biological VOCs from real samples.
 | ||
| Fig. 2 Schematic of the sampling device for on-line human breath sampling coupled with PTR/MS detection.48 | ||
Development of sampling techniques for biological VOCs has always been a forefront for bioodoromics. With the development and technical innovations in field of extraction media, sampling devices and in-depth understanding of bio-information among biological VOCs, it will primarily pave a path for adequate preconditions and a solid base for bioodoromics in the future.
3. Analytical techniques
The entire information of biological VOC composition including species and contents of biological VOCs during metabolic processes is required for the study of consequent bio-information. In most cases, biological VOC composition is complicated at trace levels. Therefore, achieving the qualitative and quantitative information of biological VOC components should mainly rely on sensitive analytical techniques with high throughput. Nowadays, the most popular and suitable analytical techniques for biological VOCs include chromatography, MS, gas sensor and spectroscopy techniques.3.1 Chromatography and mass spectrometry
GC is an excellent chromatographic technique for the separation and analysis of gas target compounds which has been considered a typical analytical technique for VOCs and biological VOCs nowadays.49–51 Separation capacity of GC procedure and sensitivity of consequent detection will directly decide the information throughput for the study of bioodoromics. To date, the development of multidimensional (MD) GC greatly improves the separation capacity of complex biological VOCs and makes the distillation of potential bio-information from corresponding VOC characteristics easier than single-dimensional chromatography.52,53 Mitrevski et al.52 developed a novel hybrid comprehensive 2D GC for precise and high resolution characterization of multiple volatile components from a coffee sample. The separation capacity and resolution could be well improved without matrix interference. After GC separation the separated biological VOCs should be detected by efficient and sensitive detectors to achieve the final analytical results with high throughput. Common detectors coupling with GC for biological VOCs mainly includes flame ionization detector (FID) and MS. GC/FID is very suitable for detecting hydrocarbons and other easily flammable VOCs across a wide range of concentrations.54 Compared with GC/FID, GC/MS has been considered as one of the most powerful tools for qualification and quantification of biological VOCs in numerous reports due to its excellent structure interpretation function, detection sensitivity and speed. Quadrupole MS is one of the most frequently used detectors in GC/MS for biological VOCs.MS is also a crucial analytical technique for biological VOCs with the function of elucidating the chemical structures of target analytes. Apart from quadrupole MS, nowadays some new MS detectors have been developed for the analysis of biological VOCs such as automated cylindrical ion trap mass spectrometer (CITMS),55 selected ion flow tube mass spectrometer (SIFTMS),56 proton-transfer reaction-linear ion trap mass spectrometer (PTRMS),57 time of flight mass spectrometer (TOFMS),58 multicapillary column ion mobility spectrometers (MCCIMS)59,60 and proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOFMS)61,62etc. These newly proposed MS techniques possess the higher detection efficiency and sensitivity for the target components successively flowing into MS. Especially, the great value of SIFTMS, PTRMS and PTR-TOFMS is their real-time facility and the instant quantification capability, which would be quite suitable for trace, unstable and short-lived biological VOCs generated during metabolism. MCCIMS is increasingly in demand for biological applications and process control due to its easily-controlled operation conditions. MCCIMS can be operated not only at room temperature (∼25 °C) but also at any ambient pressure. MICCIMS also does not require any specialty gases such as argon, helium or nitrogen. Moreover, the sampling and acquisition time is shorter than that of conventional MS protocol. Thus, MCCIMS is very suitable for the rapid, on-site and in-line analysis of biological VOCs even in complex and humid gas samples such as human breath.59,60 However, until today these novel MS detectors have only been applied for the study of simple volatile biomarkers such as propane, acetone, isoprene, methanol, carbon disulfide, 2-propenal, ethylbenzene and isopropyl alcohol56–58 in human exhaled breath samples whose matrices are relatively simple.
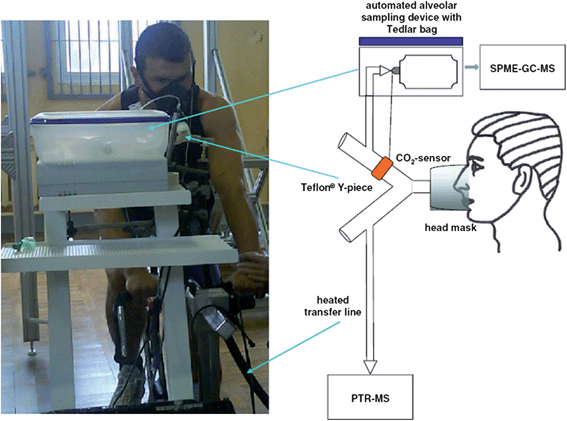
Herbig et al.61 presented the first on-line breath measurements by PTR-TOFMS which combined with buffered end-tidal sampling technique. They showed this technique could measure all ionized VOCs up to 320 Th from one single exhalation with a limit-of-detection at sub-pg level. They successfully exploited the high mass resolving power of PTR-TOFMS for the on-line identification of the main breath components of several healthy volunteers. Maddula et al.59 developed a MCCIMS method for the determination of microbial VOCs and characterization of metabolic activity of growing Escherichia coli. MCCIMS chromatogram of the microbial culture off-gas of the acetone-producing E. coli strain BL21 pLB4 revealed four analytes that positively correlated with growth, which were identified as ethanol, propanone (acetone), heptan-2-one, and nonan-2-one. Hryniuk et al.56 established a new TD-SIFTMS method for the detection of acetone and isoprene in human breath and achieved a comparable result with TD-GC method. If the sampling volume can be carefully controlled, this method may be really used for simple disease diagnosis in those clinical situations when only breath analysis could be employed. Rudnicka et al.58 developed an analytical method for human exhaled VOCs by SPME-GC/TOFMS and achieved excellent limits of detection in the range of 0.31 to 0.75 ng mL−1. Propane, carbon disulfide, 2-propenal, ethylbenzene and isopropyl alcohol were tentatively considered as potential biomarkers for non-intrusive screening of lung cancer. Fig. 3 shows a typical use of SIFTMS for the detection of potential biomarkers in human breath.63 This method allowed the direct breath sampling into SIFTMS instrument and possessed excellent anti-interference capability and selectivity. However, due to the lack of suitable separation process, these MS detectors are affected by the water vapor in breath samples sometimes, which would arouse the decrease of detection sensitivity and throughput. If these new MS detectors can be coupled with GC or some other separation techniques, it would further reveal their superiority and expand their application range for biological VOCs from more real biological samples. Moreover, the development of miniaturized mass spectrometers suitable for the portable GC would facilitate the on-site analysis of biological VOCs.
 | ||
| Fig. 3 Schematic diagram of SIFTMS detection of breath volatiles by direct breath sampling.63 | ||
3.2 Gas sensor and spectroscopy
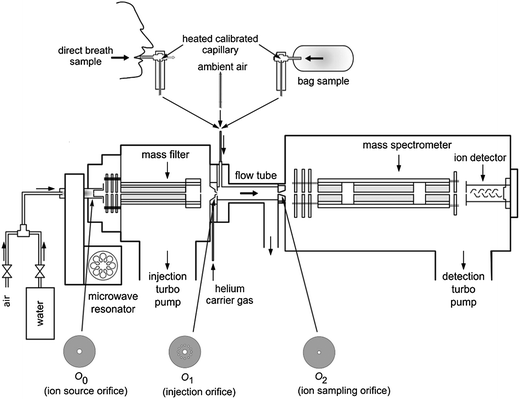
Gas sensor technique is a rapidly evolving field, driven by the increasing demand for fast online detection of VOCs including biological VOCs. Especially, electrochemical gas sensor is extremely suitable for the sensitive detection of volatile biomarkers with known chemical structures64,65 due to its low detection limits (usually at ng level). However, electrochemical gas sensors have no separation and qualitative function with weak anti-interference capability and selectivity. Therefore, usually off-line pre-separation procedures should be conducted to achieve the individually separated volatile biomarkers for the consequent gas sensor detection. Nowadays, electrochemical gas sensors have been applied for the analysis of known biological VOC components from some simple gas samples such as breath samples,66,67 but they are still not suitable for entire analysis of trace biological VOCs from complicated samples. Thus, many novel electrochemical gas sensors have been developed for the detection of important volatile biomarkers with higher detection sensitivity. Haick and his co-workers68,69 developed a series of novel nanoarray-material-based sensors for identifying and distinguishing different cancers based on the analysis of various profile characteristics of human exhaled VOCs which represent the “fingerprint” characteristics that indicate the presence of diseases. Especially, an array of sensors based on gold nanoparticles proposed by this group69 could rapidly distinguish the breath of lung cancer patients from that of healthy individuals in an atmosphere of high humidity (Fig. 4). Their results showed that sensors based on gold nanoparticles could form the basis of an inexpensive and non-invasive diagnostic tool for lung cancer. Electronic nose (e-nose) is another type of typical electrochemical gas sensor and possesses the capability of mimicking human senses using sensor arrays and pattern recognition systems. E-nose has been widely applied in the flavor or odor recognition. Most e-nose applications for bioodoromics are related to food industry, which facilitate relevant fields such as fruit quality control, food safety and flavors study based on the interpretation of food flavor or aroma characteristics.70 However, without separation steps e-nose can only generate signal patterns of biological volatile profile characteristics, but no response to every individual VOC component. Thus, it is hardly used for the determination of volatile biomarkers during metabolism nowadays. | ||
| Fig. 4 Illustration of the diagnosis of lung cancer using breath testing.69 (a) A photograph of the array of sensors; (b) testing the exhaled breath collected from patients and simulated breath using the array of gold nanoparticle sensors. | ||
Spectroscopic techniques have created new opportunities for gas analysis in recent years. Spectroscopic detection with increasing sensitivity and analytical speed nowadays offers particular potential for the real-time analysis of trace biological VOCs. Although spectroscopic techniques have no separation function similar as gas sensors, some spectroscopic techniques such as infrared (IR) and ultraviolet (UV) spectrometry have been applied for the noninvasive analysis of trace volatile components in relatively simple exhaled breath samples.71,72 The target biological VOCs involved C2H6, NO, CO2, C2H2, etc. Baum et al.72 developed a new, miniaturized, noninvasive instrument based on dispersive UV absorption spectroscopy for rapid acetylene analysis in breath gas. The analyzer could offer fast (∼276 ms) and interference-free detection of acetylene, which may be a promising method for human breath analysis in a clinical setting. If spectroscopic techniques can be further miniaturized and coupled with suitable separation techniques, spectroscopic techniques will be one of the best choices for the on-site analysis of trace biological VOCs from more complicated samples. It can be seen that many trace biological VOCs containing crucial bio-information are instantly emerged-and-disappeared during metabolism. Therefore, it is essential to develop the accurate, sensitive, portable, rapid and easy-operating analytical techniques to catch the original bio-information for the study of bioodoromics in the future.
4. Interpretation of tentative bio-information
Developing sampling and analytical techniques allows us to obtain much more composition information on biological VOCs. However, the study of biological VOCs focuses on not only the determination of biological volatile components but also the interpretation of biological VOC characteristics and further distillation of potential bio-information from the high number of statistical experimental data. The connection of biological VOCs and specific metabolism pathways would raise the study of biological VOCs to a higher level, bioodoromics.73 With the aid of chemometric methods such as principal component analysis (PCA),74 normalization,75 partial least squart,76 common model strategy77 and artificial neural network,78,79 tentative bio-information has been successfully interpreted, which greatly benefits the insect prevention, disease diagnosis, criminal track-down, agricultural product quality control and food safety push the progress of bioodoromics. Especially, the combination of normalization, PCA and common model strategy has been proven to be an effective data-processing mode for the analysis of biological VOCs, which possesses much higher efficiency for the distillation of potential bio-information than any individual methods.73Firstly, insects usually release specific VOCs for communication which would greatly influence their behavior. Some important insect volatile biomarkers such as 3-octanone, 2-ethyl hexanol, 1-octanol, nonanal9 and 2-(undecyloxy)-ethanol80 have been tentatively identified and investigated using analytical methods combined with insect behavior. These typical biological VOCs are recognized as potential biomarkers for insect behavior such as food-finding, pairing-up and danger-alarming. Although the formation mechanism of insect volatile biomarkers is unclear now, these works greatly benefit insect prevention. Secondly, the relation between the change of human body VOCs and disease diagnosis has aroused great interest. Especially, some potential but crucial biomarkers such as propane, carbon disulfide, 2-propenal, ethylbenzene, isopropyl alcohol,58 hexanal, 5-methyl-undecane,64 styrene, hexadecane,65 carbon monoxide,67 isoprene, benzonitrile, octadecane and undecane81 in human exhaled breath have received much attention, since human exhaled breath has been considered as a main source of human VOCs and may directly reflect the inner metabolism status of the human body. The composition of exhaled breath can vary depending on the types of illnesses.82 Analysis of breath constituents may offer assistance in identifying potential markers, which may possess the possibility to be real early warning markers for some common diseases such as lung cancer,58 multiple sclerosis,64 Parkinson's disease,65 asthma67 and chronic obstructive pulmonary disease.81 Thirdly, many agricultural products possess different aroma profile characteristics at different growth or post harvest storage phases. The Zhang and Li group investigated aroma profile characteristics of several fruits at different storage phases and of several mango and Allium varieties at different growth phases and achieved potential but useful information for agricultural product post harvest and storage.77 Moreover, they focused on the study of volatile profile characteristics of seafood samples including oyster, razor clam, redspot swimming crab and prawn at different storage phases in order to develop a new flavor evaluation method for seafood freshness.83,84 Since seafood volatile profile characteristics always changed greatly prior to the appearance of traditional chemical marker trimethylamine during storage, the different seafood volatile profile characteristics could reflect the transitional changing seafood freshness and provide more precise warning information for seafood spoilage during storage than any single chemical markers.
Various biological VOC systems possess different and complicated volatile composition and profile characteristics. Interpretation of potential but important bio-information behind biological VOCs is a core goal of bioodoromics. Although some preliminary works for distilling potential volatile biomarkers have been reported, the further study of bioodoromics should focus on further confirmation of potential volatile biomarkers and interpretation of the relation between biological volatile characteristics or biomarkers and specific metabolism processes.
5. Outlook
As crucial bio-information carriers, it is imperative to obtain the complete composition information of biological VOCs during metabolism. Lack of efficient analytical methodology for biological VOCs hinders the progress of potential bioodoromics due to the possible loss of biological VOC components. Thus, development of the analytical methodology for biological VOCs including novel sampling techniques with high selectivity and capacity and sensitive analytical techniques with high separation and detection throughput would be a crucial trend for the study of biological VOCs. Firstly, it is noted that novel extraction media should be introduced to establish specific sampling techniques for biological VOCs, which would greatly improve the sampling selectivity and capacity. Secondly, multidimensional chromatographic techniques and some latest MS techniques should be used in the new analytical techniques for biological VOCs, which would facilitate the enhancement of separation and detection throughput. Thirdly, after the adequate and solid compositional base of biological VOCs is achieved, the relationship between biological VOC characteristics and corresponding pathways should be further interpreted and specified by efficient data processing methods, which will bring the study of biological VOCs to a higher level, namely bioodoromics.On the other hand, biological samples and target biological VOCs nowadays are mainly odor-active biological VOCs and relatively simple-matrix samples such as human breath samples. It is another important trend to expand the application range including the accumulation of more basic data of non-odor-active biological VOCs containing crucial bio-information from more biological samples with complicated sample matrixes. All in all, although the connotation and extension of bioodoromics has become clearer, there is still a long way to go from the simple qualification and quantification of biological VOCs to real bioodoromics in the future. It is high time to recognize and embrace the power of bioodoromics, generated as a new interdisciplinary research field of analytical chemistry, biochemistry, food and agricultural science, which would lead to a major advance in related scientific fields such as insect prevention, disease diagnosis, criminal track-down, agricultural product quality control and food safety in the future.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (no. 21275168 and no. 21127008) and the Fundamental Research Funds for the Central Universities (no. 11lgpy102) for financially supporting this research.References
- E. A. Hallem, N. A. Fox, L. J. Zwiebel and J. R. Carlson, Nature, 2004, 427, 212–213 CrossRef CAS.
- J. M. Mateo, Proc. R. Soc. London, Ser. B, 2002, 269, 721–727 CrossRef.
- J. Kessekmeier and M. Staudt, J. Atmos. Chem., 1999, 33, 23–88 CrossRef.
- T. Kida, T. Doi and K. Shimanoe, Chem. Mater., 2010, 22, 2662–2667 CrossRef CAS.
- L. C. Zhang, Q. Zhou, Z. H. Liu, X. D. Hou, Y. B. Li and Y. Lv, Chem. Mater., 2009, 21, 5066–5071 CrossRef CAS.
- L. Wang, A. Teleki, S. E. Pratsinis and P. I. Gouma, Chem. Mater., 2008, 20, 4794–4796 CrossRef CAS.
- O. S. Kwon, J. Y. Hong, S. J. Park, Y. J. Jang and J. Jang, J. Phys. Chem. C, 2010, 114, 18874–18879 CAS.
- D. Wang, Q. T. Wang, Z. M. Zhang and G. N. Chen, Analyst, 2012, 137, 476–480 RSC.
- Z. M. Zhang, Q. T. Wang and G. K. Li, Anal. Chim. Acta, 2012, 727, 13–19 CrossRef CAS.
- D. Wang, Z. M. Zhang, L. Luo, T. M. Li, L. Zhang and G. N. Chen, Nanotechnology, 2009, 20, 465–702 Search PubMed.
- K. Hosono, I. Matsubara, N. Murayama, S. Woosuck and N. Izu, Chem. Mater., 2005, 17, 349–354 CrossRef CAS.
- Y. Zilberman, U. Tisch, G. Shuster, W. Pisula, X. L. Feng, K. Müllen and H. Haick, Adv. Mater., 2010, 22, 4317–4320 CrossRef CAS.
- D. Barreca, E. Comini, A. P. Ferrucci, A. Gasparotto, C. Maccato, C. Maragno, G. Sberveglieri and E. Tondello, Chem. Mater., 2007, 19, 5642–5649 CrossRef CAS.
- X. Q. Qiu, M. Miyauchi, K. Sunada, M. Minoshima, M. Liu, Y. Lu, D. Li, Y. Shimodaira, Y. Hosogi, Y. Kuroda and K. Hashimoto, ACS Nano, 2012, 6, 1609–1618 CrossRef CAS.
- Z. Y. Gu, C. X. Yang, N. Chang and X. P. Yan, Anal. Chem., 2012, 45, 734–745 CAS.
- D. Bradshaw, A. Garai and J. Huo, Chem. Soc. Rev., 2012, 41, 2344–2381 RSC.
- Z. Y. Gu, G. Wang and X. P. Yan, Anal. Chem., 2010, 82, 1365–1370 CrossRef CAS.
- N. Chang, Z. Y. Gu, H. F. Wang and X. P. Yan, Anal. Chem., 2011, 83, 7094–7101 CrossRef CAS.
- S. S. Pandit, H. G. Chidley, R. S. Kulkarni, K. H. Pujari, A. P. Giri and V. S. Gupta, Food Chem., 2009, 114, 363–372 CrossRef CAS.
- S. Lee, K. Umano, T. Shibamoto and K. Lee, Food Chem., 2005, 91, 131–137 CrossRef CAS.
- M. A. Ferhat, N. Tigrine-Kordjani, S. Chemat, B. Y. Meklati and F. Chemat, Chromatographia, 2007, 65, 217–222 CAS.
- B. K. Huang, L. P. Qiu, Q. C. Chu, Q. Y. Zhang, L. H. Gao and H. C. Zheng, Chromatographia, 2009, 69, 489–496 CAS.
- A. Colomb, N. Yassaa, J. Williams, I. Peeken and K. Lochte, J. Environ. Monit., 2008, 10, 325–330 RSC.
- V. D. Preter, G. V. Staeyen, D. Esser, P. Rutgeerts and K. Verbeke, J. Chromatogr., A, 2009, 1216, 1476–1483 CrossRef.
- A. C. Soria, I. Martínez-Castro and J. Sanz, Food Res. Int., 2008, 41, 838–848 CrossRef CAS.
- A. C. Soria, I. Martínez-Castro and J. Sanz, J. Chromatogr., A, 2009, 1216, 3300–3304 CrossRef CAS.
- G. F. Ouyang, D. Vuckovic and J. Pawliszyn, Chem. Rev., 2011, 111, 2784–2814 CrossRef CAS.
- M. Kusano, E. Mendez and K. G. Furton, Anal. Bioanal. Chem., 2011, 400, 1817–1826 CrossRef CAS.
- E. M. Sheehan, M. A. Limmer, P. Mayer, U. G. Karlson and J. G. Burken, Environ. Sci. Technol., 2012, 46, 3319–3325 CrossRef CAS.
- A. Prieto, O. Basauri, R. Rodil, A. Usobiaga, L. A. Fernández, N. Etxebarria and O. Zuloaga, J. Chromatogr., A, 2010, 1217, 2642–2666 CrossRef CAS.
- A. Buettner, Flavour Fragrance J., 2007, 22, 465–473 CrossRef CAS.
- A. Kloskowski and M. Pilarczyk, Anal. Chem., 2009, 81, 7363–7367 CrossRef CAS.
- R. Q. Yang and W. L. Xie, Forensic Sci. Int., 2004, 139, 177–181 CrossRef CAS.
- I. Bruheim, X. C. Liu and J. Pawliszyn, Anal. Chem., 2003, 75, 1002–1010 CrossRef CAS.
- X. Li, G. F. Ouyang, H. Lord and J. Pawliszyn, Anal. Chem., 2010, 82, 9521–9527 CrossRef CAS.
- Y. Gong, I. Y. Eom, D. W. Lou, D. Hein and J. Pawliszyn, Anal. Chem., 2008, 80, 7275–7282 CrossRef CAS.
- C. F. Duan, Z. Shen, D. P. Wu and Y. F. Guan, TrAC, Trends Anal. Chem., 2011, 30, 1568–1574 CrossRef CAS.
- H. L. Lord, W. Q. Zhan and J. Pawliszyn, Anal. Chim. Acta, 2010, 677, 3–18 CrossRef CAS.
- M. Mieth, S. Kischkel, J. K. Schubert, D. Hein and W. Miekisch, Anal. Chem., 2009, 81, 5851–5857 CrossRef CAS.
- D. J. Penn, E. Oberzaucher, K. Grammer, G. Fischer, H. A. Soini, D. Wiesler, M. V. Novotny, S. J. Dixon, Y. Xu and R. G. Brereton, J. R. Soc., Interface, 2007, 4, 331–340 CrossRef.
- S. Riazanskaia, G. Blackburn, M. Harker, D. Taylor and C. L. P. Thomas, Analyst, 2008, 133, 1020–1027 RSC.
- M. Mieth, J. K. Schubert, T. Gröger, B. Sabel, S. Kischkel, P. Fuchs, D. Hein, R. Zimmermann and W. Miekisch, Anal. Chem., 2010, 82, 2541–2551 CrossRef CAS.
- M. Alonso, M. Castellanos, E. Besalú and J. M. Sanchez, J. Chromatogr., A, 2012, 1252, 23–30 CrossRef CAS.
- Z. M. Zhang, J. J. Cai, G. H. Ruan and G. K. Li, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2005, 822, 244–252 CrossRef CAS.
- H. J. Martin, S. Riazanskaia and C. L. Paul Thomas, Analyst, 2012, 137, 3627–3634 RSC.
- J. P. Spinhirne, J. A. Koziel and N. K. Chirase, J. Chromatogr., A, 2004, 1025, 63–69 CrossRef CAS.
- C. A. Zini, F. Augusto, E. Christensen, B. P. Smith, E. B. Caramão and J. Pawliszyn, Anal. Chem., 2001, 73, 4729–4735 CrossRef CAS.
- H. Schwoebel, R. Schubert, M. Sklorz, S. Kischkel, R. Zimmermann, J. K. Schubert and W. Miekisch, Anal. Bioanal. Chem., 2011, 401, 2079–2091 CrossRef CAS.
- G. A. Eiceman, H. H. Hill, Jr and J. Gardea-Torresdey, Anal. Chem., 1998, 70, 321R–339R CrossRef CAS.
- E. Matisová and M. Dömötörová, J. Chromatogr., A, 2003, 1000, 199–221 CrossRef.
- N. H. Snow and G. P. Bullock, J. Chromatogr., A, 2010, 1217, 2726–2735 CrossRef CAS.
- B. Mitrevski and P. J. Marriott, Anal. Chem., 2012, 84, 4837–4843 CrossRef CAS.
- P. J. Marriott, S. T. Chin, B. Maikhunthod, H. G. Schmarr and S. Bieri, TrAC, Trends Anal. Chem., 2012, 34, 1–21 CrossRef CAS.
- R. W. Current, E. I. Kozliak and A. J. Borgerding, Environ. Sci. Technol., 2001, 35, 1452–1457 CrossRef CAS.
- G. D. Edwards, P. B. Shepson, J. W. Grossenbacher, J. M. Wells, G. E. Patterson, D. J. Barket, Jr, S. Pressley, T. Karl and E. Apel, Anal. Chem., 2007, 79, 5040–5050 CrossRef CAS.
- A. Hryniuk and B. M. Ross, Int. J. Mass Spectrom., 2009, 285, 26–30 CrossRef CAS.
- M. E. O. Hara, S. O. Hehir, S. Green and C. A. Mayhew, Physiol. Meas., 2008, 29, 309–330 CrossRef.
- J. Rudnicka, T. Kowalkowski, T. Ligor and B. Buszewski, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2011, 879, 3360–3366 CrossRef CAS.
- S. Maddula, L. M. Blank, A. Schmid and J. I. Baumbach, Anal. Bioanal. Chem., 2009, 394, 791–800 CrossRef CAS.
- M. Jünger, B. Bödeker and J. I. Baumbach, Anal. Bioanal. Chem., 2010, 396, 471–482 CrossRef.
- J. Herbig, M. Müller, S. Schallhart, T. Titzmann, M. Graus and A. Hansel, J. Breath Res., 2009, 3, 027004 CrossRef.
- M. Graus, M. Müller and A. Hansela, J. Am. Soc. Mass Spectrom., 2010, 21, 1037–1044 CrossRef CAS.
- D. Smith and P. Španěl, TrAC, Trends Anal. Chem., 2011, 30, 945–959 CrossRef CAS.
- R. Ionescu, Y. Broza, H. Shaltieli, D. Sadeh, Y. Zilberman, X. L. Feng, G. L. Marmor, I. Lejbkowicz, K. Müllen, A. Miller and H. Haick, ACS Chem. Neurosci., 2011, 2, 687–693 CrossRef CAS.
- U. Tisch, Y. Aluf, R. Ionescu, M. Nakhleh, R. Bassal, N. Axelrod, D. Robertman, Y. Tessler, J. P. M. Finberg and H. Haick, ACS Chem. Neurosci., 2012, 3, 161–166 CrossRef CAS.
- S. Thanachasai, S. Rokutanzono, S. Yoshida and T. Watanabe, Anal. Sci., 2002, 18, 773–777 CrossRef CAS.
- W. Zetterquist, H. Marteus, M. Johannesson, S. L. Nordvall, E. Ihre, J. O. N. Lundberg and K. Alving, Eur. Respir. J., 2002, 20, 92–99 CrossRef CAS.
- U. Tisch and H. Haick, MRS Bull., 2010, 35, 797–804 CrossRef CAS.
- G. Peng, U. Tisch, O. Adams, M. Hakim, N. Shehada, Y. Y. Broza, S. Billan, R. A. Bortnyak, A. Kuten and H. Haick, Nat. Nanotechnol., 2009, 4, 669–673 CrossRef CAS.
- A. H. Gómez, J. Wang, G. X. Hu and A. G. Pereira, J. Food Eng., 2008, 85, 625–631 CrossRef.
- C. Roller, K. Namjou, J. Jeffers, W. Potter, P. J. McCann and J. Grego, Opt. Lett., 2002, 27, 107–109 CrossRef CAS.
- M. M. Baum, S. Kumar, A. M. Lappas and P. D. Wagner, Rev. Sci. Instrum., 2003, 74, 3104–3110 CrossRef CAS.
- Z. M. Zhang and G. K. Li, Microchem. J., 2010, 95, 127–139 CrossRef CAS.
- N. Bhattacharyya, S. Seth, B. Tudu, P. Tamuly, A. Jana, D. Ghosh, R. Bandyopadhyay and M. Bhuyan, J. Food Eng., 2007, 80, 1146–1156 CrossRef CAS.
- C. H. Deng, X. M. Zhang, W. M. Zhu and J. Qian, Anal. Bioanal. Chem., 2004, 378, 518–522 CrossRef CAS.
- P. Jonsson, J. Gullberg, A. Nordström, M. Kusano, M. Kowalczyk, M. Sjöström and T. Moritz, Anal. Chem., 2004, 76, 1738–1745 CrossRef CAS.
- Z. M. Zhang and G. K. Li, Microchem. J., 2007, 86, 29–36 CrossRef CAS.
- A. Bockreis and J. Jager, Environ. Model. Software, 1999, 14, 421–426 CrossRef.
- P. Ciosek, Z. Brzózka, W. Wróblewski, E. Martinelli, C. D. Natale and A. D. Amico, Talanta, 2005, 67, 590–596 CrossRef CAS.
- S. A. Teale, J. D. Wickham, F. P. Zhang, J. Su, Y. Chen, W. Xiao, L. M. Hanks and J. G. Millar, J. Econ. Entomol., 2011, 104, 1592–1598 CrossRef CAS.
- J. J. B. N. V. Berkel, J. W. Dallinga, G. M. Möller, R. W. L. Godschalk, E. J. Moonen, E. F. M. Wouters and F. J. V. Schooten, Respir. Med., 2010, 104, 557–563 CrossRef.
- B. Buszewski, J. Rudnicka, T. Ligor, M. Walczak, T. Jezierski and A. Amann, TrAC, Trends Anal. Chem., 2012, 38, 1–12 CrossRef CAS.
- Z. M. Zhang, T. L. Li, D. Wang, L. Zhang and G. N. Chen, Food Chem., 2009, 115, 1150–1157 CrossRef CAS.
- Z. M. Zhang, G. K. Li, L. Luo and G. N. Chen, Anal. Chim. Acta, 2010, 659, 151–158 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
