The development of quinolone esters as novel antimalarial agents targeting the Plasmodium falciparum bc1 protein complex
Robin
Cowley
a,
Suet
Leung
a,
Nicholas
Fisher
b,
Mohammed
Al-Helal
b,
Neil G.
Berry
a,
Alexandre S.
Lawrenson
a,
Raman
Sharma
a,
Alison E.
Shone
b,
Stephen A.
Ward
b,
Giancarlo A.
Biagini
*b and
Paul M.
O′Neill
*a
aDepartment of Chemistry, University of Liverpool, Liverpool, L69 7ZD, UK. E-mail: pmoneill@liv.ac.uk; Tel: +44 (0)1517943553
bLiverpool School of Tropical Medicine, Pembroke Place, Liverpool, L3 5QA, UK. E-mail: biagini@liv.ac.uk; Tel: +44 (0)151 705 3151
First published on 1st September 2011
Abstract
Using the Gould-Jacobs methodology a small array of 6- and 7-substituted quinolones have been prepared. Analogues in the 7-series express activity as low as 0.46 nM versus Plasmodium falciparum malaria parasites and docking studies performed in silico at the yeast Qo site demonstrate a key role for residues His182 and Glu 272 in the recognition of high potency inhibitors.
In the 21st century malaria continues to be one of the most deadly infectious diseases in the world.1 Current estimates place 40% of the global population at risk of malarial infection on a daily basis and the World Health Organisation attributes 5.2% of deaths in low-income countries to malaria.2Plasmodium falciparum is the most prevalent of the four parasite strains affecting mankind; it is also the most deadly being responsible for around 90% of the 1 million fatalities attributed to malaria annually.3 With 85% of P. falciparum infections now resistant to at least one frontline treatment,4 and the emergence of resistance to artemisinin-based therapies,5 the need for new antimalarial agents grows ever more urgent.
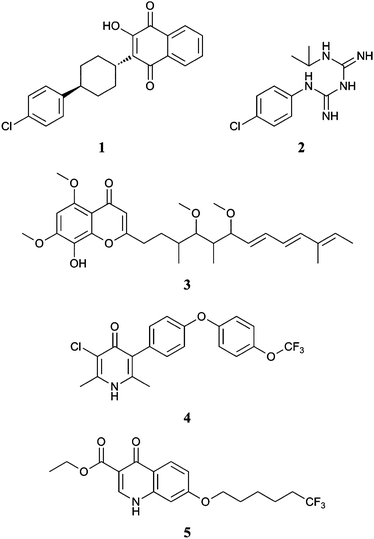
In 2000 the drug preparation Malarone® was released by GlaxoSmithKline for the treatment and prevention of multidrug resistant malaria.6 The active ingredients of Malarone® are atovaquone 1 and proguanil 2.7 Atovaquone is a ubiquinone (CoQ) competitive inhibitor, specifically inhibiting plasmodium mitochondrial bc1 activity,8 validating this enzyme complex as a target for development of novel antimalarial preparations.9
The bc1 complex is a homodimeric transmembrane protein with a molecular mass of 240 kDa. The electron-transferring core of the enzyme includes three catalytic subunits (cytochrome b, the Rieske iron-sulphur protein (ISP), and cytochrome c1) that catalyse the transfer of electrons from ubiquinol to cytochrome c coupled to the vectorial translocation of protons across the inner mitochondrial membrane.10 Loss of bc1 activity results in a loss of mitochondrial function as evidenced by the collapse of the trans-membrane electrochemical potential.11 It is believed that in asexual parasites, one of the essential functions of the mitochondrion is to provide orotate for the biosynthesis of pyrimidine through the activity of dihydroorotate dehydrogenase (DHODH).12
Generation of the membrane potential is achieved by a complex bifurcated redox pathway (the protonmotive ‘Q-cycle’)10,13 involving the endogenous compounds ubiquinol and ubiquinone, the net result of which is the release of two protons to the cytosolic side of the inner mitochondrial membrane per two electrons transferred from ubiquinol to cytochrome c.10
The target active site in this study is the Qo site at which ubiquinol is oxidized. The Qo site is located at the interface of two four-helical bundles in the cytochrome b subunit, residing in-between the bL heme and the 2Fe–2S cluster of the iron-sulphur protein when the latter is docked in ISP docking crater. This mainly hydrophobic pocket of approximately 15 Å in length, which has the shape of a saddle,14 is also believed to be the target site a number of pesticides15 as well as that of atovaquone, as evidenced by mutation studies in model organisms, e.g.,16 identification of point mutations in atovaquone/Malarone resistant parasites17 and molecular modelling studies.18 The Qo site is also known to be the site of action of the natural bc1 inhibitor stigmatellin 3.
It has been shown through crystallographic and spectrophotometric studies that Qo site inhibitors can be categorised as either those that promote movement or fix the position of the water-soluble domain of Rieske iron-sulphur protein.14 Molecules that contain a chromone or quinone analogues,7 (compounds 1–5) fix the position of the water-soluble head group of the Rieske iron-sulphur protein. The key interactions are linked directly to the structure of the active site. A polar head group is required to form hydrogen bond associations within the binding pocket, connected to an alkyl or aryl chain that lies along a cylindrical hydrophobic pocket. The rationale behind a quinolone template was that it will provide the polar head group required for a bc1 inhibitor as evidenced by recent work by Riscoe et al. on quinolone and acridone templates which has identified a number of compounds that are highly potent against P. falciparum.19 However their structures contained long, flexible perfluorinated alkyl side-chains that do not provide drug-like templates 5. Our aim is to identify suitable aromatic alternatives that have the potential to be taken forward as drug leads. In our current work we have synthesised and measured the antimalarial activity against P. falciparum of two series of novel quinolones, substituted at positions 6 and 7 respectively.
A potential advantage of the quinolone template is that, when substituted appropriately, it can yield compounds with the ability to chelate haem by π-stacking, in a similar manner to the 4-aminoquinolines.20 This allows the potential for a single drug compound capable of attacking the parasite by two separate mechanisms, a feature that may hinder the development of resistance.
Twenty novel quinolones were developed as two parallel series based on a single core template (Fig. 1). Both series were designed with a polar ‘head’ group at the 3 position and then an aryl ‘tail’ extending from either the 6 position or the 7 position depending on the series.
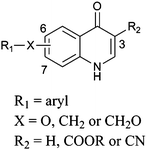 | ||
| Fig. 1 Showing the generic template for our target quinolones. | ||
Synthesis of the library was achieved by application of the Gould-Jacobs protocol21 to appropriately substituted anilines (Scheme 1). para-Substituted anilines giving rise to the 6 series, 6–15a, and meta-anilines the 7 series, 6–15b. Variation of the aniline substitution allowed us to explore the structure activity relationship in the hydrophobic channel, testing the effect of a phenoxy linker versus a methylene or benzyloxy linker in the aryl chain and the effect that having a trifluoromethoxy group present had on activity. Similarly use of ethyl (ethoxymethylene) cyanoacetate (Scheme 2)22 in place of diethyl ethoxymethylenemalonate allowed us to test the influence of a nitrile group at the 3 position rather than an ester and decarboxylation (Scheme 3) allowed us to determine whether the quinolone ring system itself was a sufficiently polar group to interact with the protein active site. Finally transesterification of the originally synthesised ethyl esters (Scheme 4) allowed the steric tolerances of the active site to be tested.23 Each of the 20 compounds was tested in vitro against cultured ‘wild-type’ 3D7 P. falciparum malarial parasites (Table 1).† This primary biological data made a number of SAR aspects readily apparent. First of all the 7 series compounds 6–15b were superior to their 6 series analogues 6–15a in almost all cases, the most active compound 10b is more than 300 times more potent than its 6 series analogue 10a. Secondly ester functionality in the 3 position is essential for good levels of activity, with the nitrile 12 and decarboxylated analogues 13 effectively rendering the molecules inactive. However the particular ester group used was equally important as the steric tolerances of the active site appeared to be quite restrictive. In the 7-series the activity of the original ethyl ester 6b was improved on by the isopropyl analogue 14b but further increases in bulk were not as well tolerated (see cyclohexyl compound 15b). In the 6-series both the larger esters exhibited a drop in potency relative to the original ethyl compound.
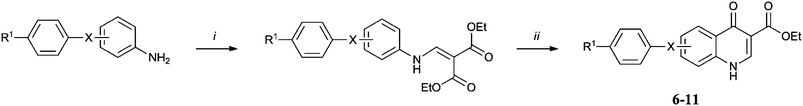 | ||
| Scheme 1 Reagents and conditions: (i) diethyl ethoxymethylenemalonate, 100 °C, 12 h; (ii) Dowtherm A, 240 °C, 1 h. | ||
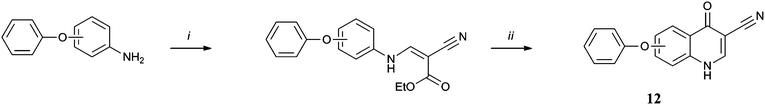 | ||
| Scheme 2 Reagents and conditions: (i) ethyl (ethoxymethylene) cyanoacetate, 120 °C, 12 h; (ii) Dowtherm A, 240 °C, 12 h. | ||
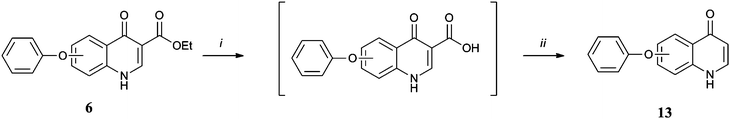 | ||
| Scheme 3 Reagents and conditions: (i) NaOHaq (1), MeOH, 100 °C, 1 h; (ii) Dowtherm A, 240 °C, 1 h. | ||
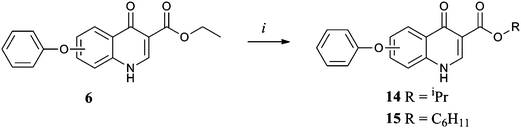 | ||
| Scheme 4 Reagents and conditions: (i) titanium(IV) isopropoxide (cat.), ROH, reflux, 24 h. | ||
| Compound | Structure | IC50vs. 3D7 (nM)a | CLogP by ALog PS2.1c |
|---|---|---|---|
| a Average value from at least 3 independent experiments. b Insufficient solubility for testing. c http://www.vcclab.org/lab/alogps. | |||
| 6a |

|
164.0 | 3.07 ± 0.41 |
| 6b |

|
19.3 | 3.07 ± 0.40 |
| 7a |

|
40.4 | 3.36 ± 0.43 |
| 7b |

|
4.57 | 3.36 ± 0.43 |
| 8a |

|
No Datab | 3.02 ± 0.45 |
| 8b |

|
No Datab | 3.02 ± 0.45 |
| 9a |

|
229.8 | 4.07 ± 0.84 |
| 9b |

|
5.30 | 4.07 ± 0.84 |
| 10a |

|
141.5 | 4.40 ± 0.80 |
| 10b |

|
0.46 | 4.41 ± 0.79 |
| 11a |

|
674.8 | 4.05 ± 0.82 |
| 11b |

|
5.00 | 4.05 ± 0.82 |
| 12a |

|
> 10 μM | 2.84 ± 0.80 |
| 12b |

|
8102.5 | 2.84 ± 0.80 |
| 13a |

|
4698.0 | 2.72 ± 0.42 |
| 13b |

|
6725.0 | 2.72 ± 0.42 |
| 14a |

|
183.9 | 3.42 ± 0.45 |
| 14b |

|
8.45 | 3.42 ± 0.45 |
| 15a |

|
1820.4 | 4.36 ± 0.67 |
| 15b |

|
29.6 | 4.36 ± 0.67 |
The nature of the aryl linker was also shown to be of significance in both series. Benzyl compounds are up to 10-fold more potent than their corresponding phenoxy and benzyloxy analogues. The inclusion of a trifluoromethoxy moiety on the aryl group was also shown to significantly boost potency in the 7 substituted series, 6bvs.9b and 7bvs.10b. However in the 6 substituted series the inclusion of trifluoromethoxy functionality was demonstrated to result in a drop in potency of up to 4 fold, 7avs.10a. Based on the exceptional potency of analogue 10b we decided to explore the activity of this molecule in the Plasmodium falciparum cytochrome c reductase assay to confirm that molecules in this class are indeed acting though bc1 inhibition. In this assay quinolone ester 10b was shown to be highly potent against the plasmodial bc1 enzyme with an EC50 of 1.3 nM which places this molecule in the same region of potency as atovaquone (3 ± 2 nM).24An atomic structure for P. falciparum bc1 is not available. In an effort to rationalise the biological observations the compounds were modelled in silico using the crystal structure of yeast bc1 protein (Protein Data Bank accession code 3CX5)25 in order to visualise the interactions between each analogue and the Qo active site. Although not identical to the parasite bc1 complex the yeast protein shares 40% homology and the Qo region is well conserved between the two proteins. The structure of the yeast bc1 complex, crystallised with stigmatellin 3 bound in the Qo site, is shown in Fig. 2a and Fig. 2b.25 Using GOLD, stigmatellin can be removed and other compounds docked in its place; using molecular mechanics the most probable binding modes of the compounds were calculated according to non-covalent interactions with the protein environment. (See Fig. 3 for compound 9b)
![(a) Stigmatellin 3 bound in the Qo site of yeast bc1 (3CX5.PDB). One catalytic unit from the homodimeric structure is highlighted, with cytochrome b, the ISP and cytochrome c1 in orange, green and purple respectively. Haem groups (cytochromes b and c1) are represented by red wireframe models, with the the ISP [2Fe2S] cluster in spacefill. The approximate position of the inner mitochondrial membrane lipid bilayer is represented in cartoon form. (b). Detail of the molecular interactions at the Qo site of stigmatellin-inhibited bc1.](/image/article/2012/MD/c1md00183c/c1md00183c-f2.gif) | ||
| Fig. 2 (a) Stigmatellin 3 bound in the Qo site of yeast bc1 (3CX5.PDB). One catalytic unit from the homodimeric structure is highlighted, with cytochrome b, the ISP and cytochrome c1 in orange, green and purple respectively. Haem groups (cytochromes b and c1) are represented by red wireframe models, with the the ISP [2Fe2S] cluster in spacefill. The approximate position of the inner mitochondrial membrane lipid bilayer is represented in cartoon form. (b). Detail of the molecular interactions at the Qo site of stigmatellin-inhibited bc1. | ||
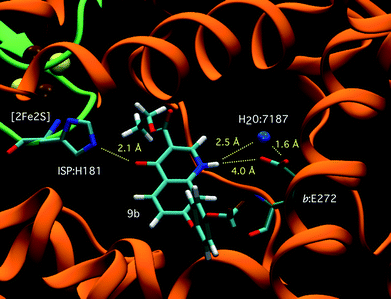 | ||
| Fig. 3 Compound 9b bound in the Qo site of yeast bc1 with residues His181 (ISP), Glu272 (cytochrome b) and H-bond interactions highlighted. Qo site Water molecule 7187 forms a H-bond bridge between Glu272 and the quinolone NH. | ||
Using this method we see that the most active compounds all adopt the same binding position within the Qo site of cytochrome b (Fig. 3) distal to haem bl and proximal to the [2Fe2S] iron-sulphur cluster of the Rieske protein. In these atomic models, the quinolone portion of the compound is oriented such that a water-mediated hydrogen bond can form between the carboxyl group of cytochrome b residue Glu272 and the quinolone N–H. A second H-bond is predicted to form between the quinolone carbonyl and protonated imidazole εN atom of ISP residue His181 (a ligand to the [2Fe2S] cluster). Stabilising hydrophobic interactions to the bound inhibitor are provided by the sidechains of cytochrome b helix ef residues Tyr279, Leu275 and Phe278, E-ef loop residue Ile269 and helix F1 residue Met295. It should be noted that Phe278 is conserved in yeast and P. falciparum b only; in mammals it is replaced by the small aliphatic residue alanine, which may help drive selectivity of these compounds.
In a similar manner to stigmatellin, the efficacy of the quinolone head group as a Qo-site inhibitor may be due to mimicking the transition state semiquinone during the catalytic turnover of the bc1 complex by simultaneously providing a H-bond donor site (to Glu272) and H-bond acceptor site (to His181).26
In addition to the SAR observations noted here the biological testing highlighted a potential problem for further development of the library as a whole. In spite of the acceptable ClogP profiles of the quinolone ester series (3.02–4.4) the majority of these compounds were poorly soluble in most solvents (<1 μM), so much so that compounds 8a and 8b proved too insoluble to test in vitro. Strong crystal packing mediated by intermolecular hydrogen bonding are possible reasons for the poor solubility observed, and this phenomena has been observed by Manetsch and co-workers in their studies of related antimalarial 3-substituted 2-methyl-4(1H)-quinolones.27
Conclusions
In conclusion we have developed SAR for a series of 6 and 7-substituted aryloxy and benzyloxy derivatives of the parent quinolone ester chemotype 5. We have identified analogues with low nanomolar activity against both the enzyme and the parasite and provided a model for the interaction of these molecules with the Qo site where residues His182 and Glu 272 play an important role in the recognition of high potency inhibitors. Whilst the activity of this series is highly promising medicinal chemistry optimisation will be required to enhance the aqueous solubility of this template and this is the focus of our current activity in this area.Notes and references
- WHO, Global Malaria Programme Brochure, in http://www.who.int/malaria/docs/GMPbrochure.pdf [Online] 2006 Search PubMed.
- (a) R. W. Snow, C. A. Guerra and J. J. Mutheu, PLoS Med., 2008, 5, 142 Search PubMed; (b) WHO, Fact sheet N°310–The 10 leading causes of death by broad income group, 2011, http://www.who.int/mediacentre/factsheets/fs310/en/index.html Search PubMed.
- S. I. Hay, E. A. Okiro and P. W. Gething, PLoS Med., 2010, 7, 1000290 Search PubMed.
- WHO, Fact sheet N°94-Malaria, 2010, http://www.who.int/mediacentre/factsheets/fs094/en/index.html Search PubMed.
- N. J. White, Science, 2008, 320, 330 CrossRef CAS.
- GlaxoSmithKline MALARONE®. http://www.malarone.com.
- C. L. Yeates, J. F. Batchelor and E. C. Capon, J. Med. Chem., 2008, 51, 2845 CrossRef CAS.
- M. Fry and M. Pudney, Biochem. Pharmacol., 1992, 43, 1545 CrossRef CAS.
- V. Barton, N. Fisher and G. A. Biagini, Curr. Opin. Chem. Biol., 2010, 14, 440 CrossRef CAS.
- (a) P. Mitchell, FEBS Lett., 1975, 59, 137 CrossRef CAS; (b) A. R. Crofts, Biochim. Biophys. Acta, Bioenerg., 2004, 1655, 77 CrossRef CAS.
- (a) I. K. Srivastava, H. Rottenberg and A. B. Vaidya, J. Biol. Chem., 1997, 272, 3961 CrossRef CAS; (b) G. A. Biagini, P. Viriyavejakul and P. M. O'Neill, Antimicrob. Agents Chemother., 2006, 50, 1841 CrossRef CAS.
- (a) D. J. Hammond, J. R. Burchell and M. Pudney, Mol. Biochem. Parasitol., 1985, 14, 97 CrossRef CAS; (b) K. K. Seymour, A. E. Yeo and K. H. Rieckmann, Ann. Trop. Med. Parasitol., 1997, 91, 603 CrossRef CAS; (c) H. J. Painter, J. M. Morrisey and M. W. Mather, Nature, 2007, 446, 88 CrossRef CAS.
- J. L. Cape, M. K. Bowman and D. M. Kramer, Trends Plant Sci., 2006, 11, 46 CrossRef CAS.
- L. Esser, B. Quinn and Y.-F. Li, J. Mol. Biol., 2004, 341, 281 CrossRef CAS.
- T. A. Link, M. Iwata, J. Bjorkman, Molecular Modelling of Inhibitors at Qi and Qo Sites in Cytochrome bc1 Complex, in Chemistry of Crop Protection, Ramos, G. V. a. G., ed. Wiley-VCH: 2003, pp 110–130 Search PubMed.
- (a) N. Fisher and B. Meunier, Pest Manage. Sci., 2005, 61, 973 CrossRef CAS; (b) T. Wenz, R. Covian and P. Hellwig, J. Biol. Chem., 2007, 282, 3977 CrossRef CAS.
- (a) M. Korsinczky, N. Chen and B. Kotecka, Antimicrob. Agents Chemother., 2000, 44, 2100 CrossRef CAS; (b) Q. L. Fivelman, G. A. Butcher and I. S. Adagu, Malar. J., 2002, 1, 1 CrossRef; (c) A. Berry, A. Senescau and J. Lelievre, Trans. R. Soc. Trop. Med. Hyg., 2006, 100, 986 CrossRef CAS; (d) L. Musset, O. Bouchaud and S. Matheron, Microbes Infect., 2006, 8, 2599 CrossRef CAS; (e) E. Schwartz, S. Bujanover and K. C. Kain, Clin. Infect. Dis., 2003, 37, 450 CrossRef.
- J. J. Kessl, S. R. Meshnick and B. L. Trumpower, Trends Parasitol., 2007, 23, 494 CrossRef CAS.
- R. W. Winter, M. K. Riscoe and J. X. Kelly, Exp. Parasitol., 2008, 118, 487 CrossRef CAS.
- A. F. G. Slater and A. Cerami, Nature, 1992, 355, 167 CrossRef CAS.
- (a) E. Stern, G. G. Muccioli and R. Millet, J. Med. Chem., 2006, 49, 70 CrossRef CAS; (b) C. G. Wang, T. Langer and P. G. Kamath, J. Med. Chem., 1995, 38, 950 CrossRef CAS; (c) T. Furuta, T. Sakai and T. Senga, J. Med. Chem., 2006, 49, 2186 CrossRef CAS.
- D. H. Boschelli, Y. D. Wang and S. Johnson, J. Med. Chem., 2004, 47, 1599 CrossRef CAS.
- E. Lager, P. Andersson and J. Nilsson, J. Med. Chem., 2006, 49, 2526 CrossRef CAS.
- G. A. Biagini, N. Fisher and N. Berry, Mol. Pharmacol., 2008, 73, 1347 CrossRef CAS.
- S. R. N. Solmaz and C. Hunte, J. Biol. Chem., 2008, 283, 17542 CrossRef CAS.
- P. R. Rich, Biochim. Biophys. Acta, Bioenerg., 2004, 1658, 165 CrossRef CAS.
- R. M. Cross, A. Monastyrskyi and T. S. Mutka, J. Med. Chem., 2010, 53, 7076 CrossRef CAS.
Footnote |
| † Preparation of Plasmodium cell-free extract: Plasmodium falciparim (3D7 strain) was grown to a minimum of 5% parasitemia (>10 flasks) before removing the media. The blood pellet was combined and the red blood cells were lysed with 0.15% Saponin (5 mL/1 mL blood). The saponin was rapidly removed by centrifugation at (4000 rpm, 5 min). The resulting free parasite material was then washed three times using RPMI media. The free parasite material was then stored at −80 °C.Measurement of cytochrome c reductase activity in P. falciparum samples Cytochrome c reductase activity measurements were assayed spectrophotometrically in 50 mM potassium phosphate (pH 7.5), 2 mM EDTA, 10 mM KCN, and 30 μM equine cytochrome c (Sigma) at room temperature. Protein concentration (Plasmodium cell-free extract) was 15 μg/ml. Cytochrome c reductase activity was initiated by the addition of decylubiquinol (50 μM). Reduction of cytochrome c was monitored in a Cary 4000 spectrophotometer at 550 versus 542 nm, and the reaction followed for four minutes. Initial rates (computer-fitted as zero-order kinetics) were measured as a function of co-enzyme Q1 concentration. Turnover rates of cytochrome c reduction were determined using ε550–542 = 18.1 mM−1 cm−1. Inhibitors of bc1 activity were added without prior incubation. DMSO in the assays did not exceed 0.3% (v/v). IC50s were calculated by using the four-parameter logistic method. |
| This journal is © The Royal Society of Chemistry 2012 |