Metallocenoids of platinum: Syntheses and structures of triangular triplatinum sandwich complexes of cycloheptatrienyl†
Tetsuro
Murahashi
*,
Kentaro
Usui
,
Ryou
Inoue
,
Sensuke
Ogoshi
and
Hideo
Kurosawa
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka, 565-0871, Japan. E-mail: tetsu@chem.eng.osaka-u.ac.jp; Fax: (+81)6 6879 7394
First published on 6th September 2010
Abstract
A series of bis-cycloheptatrienyl triangular triplatinum complexes are synthesized as a novel class of organoplatinum sandwich compounds. The tris-ethylene triplatinum sandwich complex and the monophenyl bis-ethylene sandwich complex of triangular triplatinum are structurally characterized by X-ray crystallographic analyses. The net assembling of three Pt0 atoms between two tropylium cations is initiated by complexation of a Pt0 moiety by a tropylium ligand, where the mononuclear cycloheptatrienyl PtII intermediate is detectable in situ by NMR. It is revealed that the choice of added ligands is crucial to the efficient formation as well as conversion of the key mononuclear cycloheptatrienyl PtII intermediate.
Introduction
Recently, we found that metal sheet sandwich complexes are isolable.1–5 A variety of cyclic unsaturated hydrocarbons, such as mono- and polycyclic arenes, cycloheptatriene, cycloheptatrienyl, cyclooctatetraene, and cyclononatetraenyl, act as the sandwich ligands for tri-, tetra-, or pentanuclear palladium sheet sandwich complexes. Notable is the stable bis-cycloheptatrienyl tripalladium sandwich framework of [Pd3(μ3-C7H7)2L3]z (L = Cl; z = −1, L = CH3CN; z = +2), which is formed by the reaction of Pd2(dba)3 and a tropylium salt [C7H7][BF4] in the presence of Cl− or CH3CN, but no mechanistic insight into the formation of the bis-cycloheptatrienyl trimetal sandwich framework has been gained. Thus, it is intriguing to know how three metal atoms are self-assembled between two tropylium planes, and whether the stable trimetal sandwich framework can be constructed by employing other transition metals. Use of platinum instead of palladium in our previous study may lead to, not only an initial step to extend the chemistry of the metal sheet sandwich complexes across the periodic table, but also an understanding of the mechanism of the formation of the trimetal sandwich framework. In general, stronger Pt–ligand bonds than Pd–ligand bonds are expected, not only in the reactant and product but in the intermediate, and thus a greater chance of detection of a reaction intermediate is expected for a Pt case than a Pd case. It should also be noted here that there is no guarantee for actual synthesis of Pt–Pt bonded complexes by simply applying a synthetic method which is successful in the Pd–Pd bonded system, especially when the stronger Pt–ligand bond plays a role in the reactant and/or intermediate stage. Indeed, a simple M–M bonded complex [MI2(CH3CN)6][BF4]2 was easily obtained for M = Pd by the redox condensation reaction of [MII(CH3CN)4][BF4]2 and M02(dba)3 in CH2Cl2/CH3CN,6 but not obtained for M = Pt. Herein, we report a new design of efficient synthesis of a series of Pt3 sandwich complexes of cycloheptatrienyl based on the analysis of the reaction course. It was revealed that the net assembling of three Pt0 atoms between two tropylium cations is initiated by complexation of a Pt0 moiety by a tropylium ligand, affording a mononuclear PtII intermediate. The ease of the formation as well as the conversion of the mononuclear PtII intermediate, being significantly dependent on the nature of ligands, works as the key to the efficient formation of the Pt3 sandwich framework.Results and discussion
Synthesis and structure
The reaction of Pt2(dba)37 (dba = 1,5-diphenyl-1,4-pentadien-3-one) and [C7H7]X (X = BF4, B(C6F5)4‡) in CD2Cl2 at 25 °C resulted in no production of the trinuclear cycloheptatrienyl sandwich complexes even after 3 days. In this case, a large amount of [C7H7]X remained intact. On the other hand, the reaction of Pt2(dba)3 with [C7H7][BF4] in the presence of CH3CN (the molar ratio [Pt]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C7H7]+
[C7H7]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CH3CN] = 3
[CH3CN] = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) smoothly afforded the bis-cycloheptatrienyl triplatinum complex bearing acetonitrile ligands at the equatorial coordination sites (Scheme 1). The triangular triplatinum sandwich complex [Pt3(μ3-C7H7)2(CH3CN)3][BF4]2 (1-CH33CN) was isolated in 69% yield after recrystallization to air- and moisture stable brown crystals. It was reported that the tripalladium sandwich complex [Pd3(μ3-C7H7)2(CH3CN)3][BF4]2 was obtained by a similar fashion.41H NMR spectra of 1-CH33CN in CD3CN showed a sharp singlet signal at δ = 4.20 ppm for the cycloheptatrienyl protons. 13C{1H} NMR spectra of 1-CH33CN in CD3NO2 showed a sharp singlet signal at δ = 59.7 ppm for the cycloheptatrienyl carbons.
15) smoothly afforded the bis-cycloheptatrienyl triplatinum complex bearing acetonitrile ligands at the equatorial coordination sites (Scheme 1). The triangular triplatinum sandwich complex [Pt3(μ3-C7H7)2(CH3CN)3][BF4]2 (1-CH33CN) was isolated in 69% yield after recrystallization to air- and moisture stable brown crystals. It was reported that the tripalladium sandwich complex [Pd3(μ3-C7H7)2(CH3CN)3][BF4]2 was obtained by a similar fashion.41H NMR spectra of 1-CH33CN in CD3CN showed a sharp singlet signal at δ = 4.20 ppm for the cycloheptatrienyl protons. 13C{1H} NMR spectra of 1-CH33CN in CD3NO2 showed a sharp singlet signal at δ = 59.7 ppm for the cycloheptatrienyl carbons.
![Formation of [Pt3(μ3-C7H7)2(CH3CN)3][BF4]2 (1-CH33CN).](/image/article/2011/SC/c0sc00269k/c0sc00269k-s1.gif) | ||
| Scheme 1 Formation of [Pt3(μ3-C7H7)2(CH3CN)3][BF4]2 (1-CH33CN). | ||
The reaction of Pt2(dba)3 and [C7H7][BF4] in the presence of ethylene (1 atm) gave a triplatinum sandwich complex bearing three ethylene ligands. Thus, the mixture was stirred for 8 h at ambient temperature, and then the counter anion exchange afforded the tris-ethylene complex [Pt3(μ3-C7H7)2(C2H4)3][B(ArF)4]2 (1′-C22H44, ArF = 3,5-(CF3)2C6H3) in 55% NMR yield (Scheme 2). However, this reaction usually gave unidentified insoluble byproducts. Alternatively, 1′-C22H44 was isolated in 93% yield from [Pt3(μ3-C7H7)2(CH3CN)3][B(ArF)4]2 (1′-CH33CN) via the ligand exchange of acetonitrile ligands with ethylene (Scheme 2). 1H NMR spectra of 1′-C22H44 in acetone-d6 showed a sharp singlet signal at δ = 4.62 ppm for the cyclohetatrienyl protons, and a sharp singlet signal at δ = 4.45 ppm with a 195Pt satellite doublet (JPt–H = 70 Hz) for the ethylene protons. 13C{1H} NMR spectra in CD2Cl2 showed a singlet signal at δ = 57.2 ppm with a slightly broad 195Pt satellite doublet (JPt–C = 13 Hz) for the cycloheptatrienyl carbons and a singlet signal at δ = 71.1 ppm with 195Pt satellite peaks (JPt–C = 146 Hz, JPt–C = 14 Hz) for the ethylene carbons.
![Formation of [Pt3(μ3-C7H7)2(C2H4)3][B(ArF)4]2 (1′-C22H44).](/image/article/2011/SC/c0sc00269k/c0sc00269k-s2.gif) | ||
| Scheme 2 Formation of [Pt3(μ3-C7H7)2(C2H4)3][B(ArF)4]2 (1′-C22H44). | ||
The tris-ethylene Pt3 sandwich complex 1′-C22H44 was structurally determined by X-ray crystallographic analysis (Fig. 1). A nearly equilateral Pt3 triangle is flanked by the cycloheptatrienyl ligands, and three equatorial coordination sites are occupied by the η2-ethylene ligands. The Pt–Pt distances (2.7930(4) Å, 2.8065(4) Å) are in the range of normal Pt–Pt bond lengths, but longer than those of the known planar triangular triplatinum clusters8 such as Pt3(μ-CO)3(PCy3)3 (2.656(2) Å, 2.653(2) Å).8d The Pt–C lengths for the Pt-ethylene coordination (2.231(5) Å, 2.229(8) Å, and 2.255(7) Å) are also longer than those of the typical Pt0- and PtII ethylene complexes9 such as Pt(C2H4)(PPh3)2 (2.106(8) Å, 2.116(9) Å)9b and the Zeise's salt K[Pt(C2H4)Cl3] (2.128(3) Å, 2.135(3) Å),9h probably due to the steric congestion around the equatorial coordination sites of the [Pt3(μ3-C7H7)2]2+ sandwich framework. The relatively high standard deviations for the C–C bond lengths of the ethylene ligands make a detailed discussion about the ethylene C–C bond lengths difficult [1.40(1) Å, 1.36(1) Å for 1′-C22H44, cf. Pt(C2H4)(PPh3)2 (1.434(13) Å); K[Pt(C2H4)Cl3] (1.375(4) Å)]. A few examples of Pt2 and Pt3 clusters bearing ethylene ligands are known,10 while it is well known that ethylene acts as an excellent ligand for either mononuclear Pt0 or PtII center.
![ORTEP drawing of [Pt3(μ3-C7H7)2(C2H4)3][B(ArF)4]2 (1′-C22H44) (30% probability ellipsoids, counter anions and solvent are omitted for clarity). Selected bond lengths (Å) and angles (deg): Pt1–Pt2 2.7930(4), Pt2–Pt2* 2.8065(4), Pt1–C2 2.158(10), Pt1–C3 2.277(6), Pt2–C4 2.170(6), Pt2–C5 2.165(8), Pt2–C6 2.342(8), Pt2–C7 2.15(1), Pt1–C10 2.231(5), Pt2–C8 2.229(8), Pt2–C9 2.255(7), C1–C2 1.42(1), C2–C3 1.42(1), C3–C4 1.432(10), C4–C5 1.42(1), C5–C6* 1.40(1), C6–C7 1.44(1), C8–C9 1.40(1), C10–C10* 1.36(1), Pt1–Pt2–Pt2* 59.841(5), Pt2–Pt1–Pt2* 60.32(1).](/image/article/2011/SC/c0sc00269k/c0sc00269k-f1.gif) | ||
| Fig. 1 ORTEP drawing of [Pt3(μ3-C7H7)2(C2H4)3][B(ArF)4]2 (1′-C22H44) (30% probability ellipsoids, counter anions and solvent are omitted for clarity). Selected bond lengths (Å) and angles (deg): Pt1–Pt2 2.7930(4), Pt2–Pt2* 2.8065(4), Pt1–C2 2.158(10), Pt1–C3 2.277(6), Pt2–C4 2.170(6), Pt2–C5 2.165(8), Pt2–C6 2.342(8), Pt2–C7 2.15(1), Pt1–C10 2.231(5), Pt2–C8 2.229(8), Pt2–C9 2.255(7), C1–C2 1.42(1), C2–C3 1.42(1), C3–C4 1.432(10), C4–C5 1.42(1), C5–C6* 1.40(1), C6–C7 1.44(1), C8–C9 1.40(1), C10–C10* 1.36(1), Pt1–Pt2–Pt2* 59.841(5), Pt2–Pt1–Pt2* 60.32(1). | ||
The acetonitrile or ethylene ligands at the equatorial coordination site of the triplatinum sandwich framework can be readily replaced with other ligands. The triangular triplatinum sandwich complex with PPh3 [Pt3(μ3-C7H7)2(PPh3)3][BF4]2 (1-PPh33) or pyridine [Pt3(μ3-C7H7)2(py)3][BF4]2 (1-py) was obtained by treatment of 1-CH33CN with PPh3 or pyridine, where the former showed a 31P NMR resonance, of which the coupling pattern is diagnostic to the triangular Pt3 framework [Pt3(PPh3)3] (1JPt–P = 4508 Hz, 2JPt–P = 226 Hz, and 3JP–P = 76 Hz) (Fig. 2).11 The triangular triplatinum sandwich complex 1-PPh33 or 1-py is remarkably stable even in the presence of excess PPh3 (5 equiv.) or pyridine (5 equiv.) in solution.
![31P{1H} NMR spectra of [Pt3(μ3-C7H7)2(PPh3)3][BF4]2 (1-PPh33) in CD2Cl2 (Inset shows the expanded view of the signals).](/image/article/2011/SC/c0sc00269k/c0sc00269k-f2.gif) | ||
| Fig. 2 31P{1H} NMR spectra of [Pt3(μ3-C7H7)2(PPh3)3][BF4]2 (1-PPh33) in CD2Cl2 (Inset shows the expanded view of the signals). | ||
While 1-PPh33 and 1-py are isolable via ligand substitution reactions, addition of PPh3 or pyridine to a mixture of Pt2(dba)3 and [C7H7][B(ArF)4] (the molar ratio [Pt]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C7H7]+
[C7H7]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPh3] = 3
[PPh3] = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) in CD2Cl2 results in no production of the trinuclear sandwich complex even after 1 day, while a mononuclear adduct [Pt(η3-C7H7)(PPh3)2][B(ArF)4] (2′-PPh33) or [Pt(C7H7)(py)2][B(ArF)4] (2′-py) was formed immediately (Scheme 3). The BF4− analogue of the mononuclear PPh3 complex [Pt(η3-C7H7)(PPh3)2][BF4] (3-PPh33) was isolated in 69% yield by the reaction of [Pt(C2H4)(PPh3)2] with [C7H7][BF4], and its structure was determined by X-ray crystallographic analysis (Fig. 3). 1H NMR spectra of 2-PPh33 in CD2Cl2 at 25 °C showed a sharp singlet signal (δ = 4.80 ppm) with a 195Pt satellite doublet (JPtH = 10 Hz). This signal was significantly broadened at −80 °C, indicating that the η3-cycloheptatrienyl ligand undergoes fast fluxional rotation in solution. The pyridine complex [Pt(C7H7)(py)2][B(ArF)4] (2′-py) showed a very broad 1H NMR signal at δ = 5.9 ppm at 25 °C. It is known that the related mononuclear adduct [Pt(C7H7)(cod)2][BF4] is formed by the reaction of a Pt0 complex Pt(cod)2 (COD = 1,5-cyclooctadiene) with [C7H7][BF4].12 We confirmed that the reaction of Pt2(dba)3 with [C7H7][B(ArF)4] (ArF = 3,5-(CF3)2C6H3) in the presence of COD in CD2Cl2 (the molar ratio [Pt]
6) in CD2Cl2 results in no production of the trinuclear sandwich complex even after 1 day, while a mononuclear adduct [Pt(η3-C7H7)(PPh3)2][B(ArF)4] (2′-PPh33) or [Pt(C7H7)(py)2][B(ArF)4] (2′-py) was formed immediately (Scheme 3). The BF4− analogue of the mononuclear PPh3 complex [Pt(η3-C7H7)(PPh3)2][BF4] (3-PPh33) was isolated in 69% yield by the reaction of [Pt(C2H4)(PPh3)2] with [C7H7][BF4], and its structure was determined by X-ray crystallographic analysis (Fig. 3). 1H NMR spectra of 2-PPh33 in CD2Cl2 at 25 °C showed a sharp singlet signal (δ = 4.80 ppm) with a 195Pt satellite doublet (JPtH = 10 Hz). This signal was significantly broadened at −80 °C, indicating that the η3-cycloheptatrienyl ligand undergoes fast fluxional rotation in solution. The pyridine complex [Pt(C7H7)(py)2][B(ArF)4] (2′-py) showed a very broad 1H NMR signal at δ = 5.9 ppm at 25 °C. It is known that the related mononuclear adduct [Pt(C7H7)(cod)2][BF4] is formed by the reaction of a Pt0 complex Pt(cod)2 (COD = 1,5-cyclooctadiene) with [C7H7][BF4].12 We confirmed that the reaction of Pt2(dba)3 with [C7H7][B(ArF)4] (ArF = 3,5-(CF3)2C6H3) in the presence of COD in CD2Cl2 (the molar ratio [Pt]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C7H7]+
[C7H7]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COD] = 3
[COD] = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) resulted in no production of the trinuclear sandwich complex, where a mononuclear complex [Pt(C7H7)(cod)][B(ArF)4] (2′-cod) exhibiting a sharp signal for the cycloheptatrienyl protons at δ = 5.54 ppm (JPt-H = 16 Hz) was formed as a major product (Scheme 3). Thus, the mononuclear adduct [Pt(η3-C7H7)L2]+ (L = PPh3 or pyridine; L2 = COD) was efficiently formed from Pt2(dba)3 and [C7H7]+ in the presence of L, but was never transformed to the corresponding trinuclear sandwich complex.
3) resulted in no production of the trinuclear sandwich complex, where a mononuclear complex [Pt(C7H7)(cod)][B(ArF)4] (2′-cod) exhibiting a sharp signal for the cycloheptatrienyl protons at δ = 5.54 ppm (JPt-H = 16 Hz) was formed as a major product (Scheme 3). Thus, the mononuclear adduct [Pt(η3-C7H7)L2]+ (L = PPh3 or pyridine; L2 = COD) was efficiently formed from Pt2(dba)3 and [C7H7]+ in the presence of L, but was never transformed to the corresponding trinuclear sandwich complex.
![Formation of [Pt(η3-C7H7)L2][B(ArF)4] (2′-PPh33, L = PPh3; 2′-py, L = pyridine; 2′-cod, L2 = COD).](/image/article/2011/SC/c0sc00269k/c0sc00269k-s3.gif) | ||
| Scheme 3 Formation of [Pt(η3-C7H7)L2][B(ArF)4] (2′-PPh33, L = PPh3; 2′-py, L = pyridine; 2′-cod, L2 = COD). | ||
![ORTEP drawing of [Pt(η3-C7H7)(PPh3)2][BF4] (2-PPh33) (30% probability ellipsoids, counter anions and solvent are omitted for clarity). Selected bond lengths (Å) and angles (deg): Pt1–P1 2.283(3), Pt1–P2 2.305(2), Pt1–C1 2.235(10), Pt1–C2 2.14(1), Pt1–C3 2.23(1), C1–C2 1.40(2), C2–C3 1.43(2), C3–C4 1.44(2), C4–C5 1.36(2), C5–C6 1.44(2), C6–C7 1.29(2), C7–C1 1.48(2).](/image/article/2011/SC/c0sc00269k/c0sc00269k-f3.gif) | ||
| Fig. 3 ORTEP drawing of [Pt(η3-C7H7)(PPh3)2][BF4] (2-PPh33) (30% probability ellipsoids, counter anions and solvent are omitted for clarity). Selected bond lengths (Å) and angles (deg): Pt1–P1 2.283(3), Pt1–P2 2.305(2), Pt1–C1 2.235(10), Pt1–C2 2.14(1), Pt1–C3 2.23(1), C1–C2 1.40(2), C2–C3 1.43(2), C3–C4 1.44(2), C4–C5 1.36(2), C5–C6 1.44(2), C6–C7 1.29(2), C7–C1 1.48(2). | ||
The monocationic phenyl triangular triplatinum complexes [Pt3(μ3-C7H7)2(Ph)L2][X] (3-CH33CN-Ph, L = CH3CN, X = BF4; 3′-C22H44-Ph, L = C2H4, X = B(ArF)4) were obtained by the reaction of 1-CH33CN or 1′-C22H44 with NaBPh4. Thus, treatment of 1-CH33CN with NaBPh4 (1 equiv.) in CH3CN at 60 °C for 6 h afforded the monocationic phenyl complex (3-CH33CN-Ph) in 78% yield (Scheme 4). Phenylation of 1′-C22H44 with NaBPh4 in CD2Cl2/CD3OD proceeded at ambient temperature to afford 3′-C22H44-Ph in ca. 70% NMR yield after 30 min. The complex 3′-C22H44-Ph was also obtained via ligand exchange from 3-CH33CN-Ph with ethylene in the presence of NaB(ArF)4. The bis-ethylene phenyl complex 3′-C22H44-Ph was structurally characterized by X-ray crystallographic analysis (Fig. 4). The Pt3 triangle is distorted to an isosceles triangle with the base Pt–Pt length (Pt2–Pt3 = 2.7570(8) Å) being shorter than the other two Pt–Pt lengths (Pt1–Pt2 2.8307(8) Å, Pt3–Pt1 2.8236(8) Å). 1H NMR analyses of 3′-C22H44-Ph showed that the ethylene ligands undergo non-dissociative fluxional rotation about the Pt–ethylene axis in CD2Cl2 solution; a sharp singlet signal for the ethylene protons at δ = 3.59 ppm with a 195Pt satellite doublet (JPt–H = 60 Hz) at 23 °C was broadened and separated into two slightly broad multiplet signals at lower temperatures (δ = 3.67 ppm, δ = 3.45 ppm at −60 °C) (Fig. 5). If the ethylene ligands lie coplanar with the Pt3 triangle in the ground state in solution just as found in the crystalline state of 3′-C22H44-Ph, the observed two resonances for ethylene protons at −60 °C are of pairs of geminal protons (protons [HA] and protons [HB] in Fig. 5). The sharp singlet signal for the cycloheptatrienyl protons did not change its shape in the temperature range.
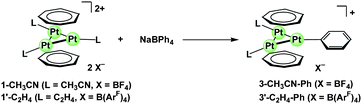 | ||
| Scheme 4 Phenylation of the triplatinum sandwich complex 1-CH33CN or 1′-C22H44 with NaBPh4. | ||
![ORTEP drawing of [Pt3(μ3-C7H7)2(Ph)(C2H4)2][B(ArF)4] (3′-C22H44-Ph). Pt1–Pt2 2.8307(8), Pt2–Pt3 2.7570(8), Pt3–Pt1 2.8236(8), Pt3–C15 2.26(1), Pt3–C16 2.26(1), Pt2–C17 2.21(2), Pt2–C18 2.22(1), Pt1–C18 2.04(2), C15–C16 1.35(2), C17–C18 1.34(2), Pt2–Pt1–Pt3 58.36(2), Pt1–Pt2–Pt3 60.69(2).](/image/article/2011/SC/c0sc00269k/c0sc00269k-f4.gif) | ||
| Fig. 4 ORTEP drawing of [Pt3(μ3-C7H7)2(Ph)(C2H4)2][B(ArF)4] (3′-C22H44-Ph). Pt1–Pt2 2.8307(8), Pt2–Pt3 2.7570(8), Pt3–Pt1 2.8236(8), Pt3–C15 2.26(1), Pt3–C16 2.26(1), Pt2–C17 2.21(2), Pt2–C18 2.22(1), Pt1–C18 2.04(2), C15–C16 1.35(2), C17–C18 1.34(2), Pt2–Pt1–Pt3 58.36(2), Pt1–Pt2–Pt3 60.69(2). | ||
![Variable temperature 1H NMR spectra of [Pt3(μ3-C7H7)2(Ph)(C2H4)2][B(ArF)4] (3′-C22H44-Ph) in CD2Cl2 (the resonances for ethylene protons are shown).](/image/article/2011/SC/c0sc00269k/c0sc00269k-f5.gif) | ||
| Fig. 5 Variable temperature 1H NMR spectra of [Pt3(μ3-C7H7)2(Ph)(C2H4)2][B(ArF)4] (3′-C22H44-Ph) in CD2Cl2 (the resonances for ethylene protons are shown). | ||
Reaction course
Here, we discuss the mechanism of the formation of the cycloheptatrienyl-Pt3 sandwich framework from Pt0 species and tropylium salts. As mentioned, the reaction of Pt2(dba)3 and [C7H7]+ in the presence of acetonitrile or ethylene afforded the desired triplatinum sandwich complexes. In the presence of PPh3, py or COD, however, no Pt3 sandwich complex but the mononuclear adduct [Pt(η3-C7H7)L2]+ (L = PPh3 or pyridine; L2 = COD) was formed efficiently, and these were never transformed to the corresponding trinuclear sandwich complexes.The mononuclear adduct [Pt(η3-C7H7)L2]+ is indeed the key intermediate in the formation of the triplatinum sandwich complex; its formation was directly detected by 1H NMR monitoring experiments when L = acetonitrile. Thus, mixing Pt2(dba)3 and [C7H7][B(C6F5)4]§ in the presence of CD3CN (the molar ratio [Pt]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C7H7]+
[C7H7]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CD3CN] = 3
[CD3CN] = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) in CD2Cl2 at 25 °C immediately afforded an initial product exhibiting a broad signal at δ = 5.50 ppm (ca. 80% after 30 min), where [C7H7][B(C6F5)4] was consumed almost quantitatively. The minor product showing a signal at δ = 4.09 ppm is the triangular triplatinum sandwich complex [Pt3(μ3-C7H7)2(CD3CN)3][B(C6F5)4]2 (1′′-CD33CN) (ca. 10% yield after 30 min). Then, the broad signal at δ = 5.50 ppm gradually decreased (ca. 70% after 2 h, ca. 30% after 1 day) while the signal of 1′′-CD33CN increased (ca. 30% after 2 h, ca. 60% after 1 day). After 5 days, the yield of 1′′-CD33CN reached ca. 80%. Thus, the product observed in the initial stage of the reaction is reasonably assumed as an intermediate in the formation of 1′′-CD33CN.
15) in CD2Cl2 at 25 °C immediately afforded an initial product exhibiting a broad signal at δ = 5.50 ppm (ca. 80% after 30 min), where [C7H7][B(C6F5)4] was consumed almost quantitatively. The minor product showing a signal at δ = 4.09 ppm is the triangular triplatinum sandwich complex [Pt3(μ3-C7H7)2(CD3CN)3][B(C6F5)4]2 (1′′-CD33CN) (ca. 10% yield after 30 min). Then, the broad signal at δ = 5.50 ppm gradually decreased (ca. 70% after 2 h, ca. 30% after 1 day) while the signal of 1′′-CD33CN increased (ca. 30% after 2 h, ca. 60% after 1 day). After 5 days, the yield of 1′′-CD33CN reached ca. 80%. Thus, the product observed in the initial stage of the reaction is reasonably assumed as an intermediate in the formation of 1′′-CD33CN.
The intermediate showing the cycloheptatrienyl proton resonance at δ = 5.5 ppm was also generated by treatment of Pt2(dba)3 with [C7H7][BF4] in CD2Cl2/CD3CN (v/v = 2/1), where a large amount of Pt2(dba)3 remained undissolved due to its low solubility in acetonitrile and thus conversion of [C7H7][BF4] was low. At lower temperatures, the broad signal at δ = 5.46 ppm further broadened and separated to three signals at −80 °C with the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 relative intensity (δ = 5.87 ppm [H3/H4 overlap], 5.58 ppm [H2], 3.27 ppm [H1]) (Fig. 6). The 13C{1H} NMR spectra at −80 °C showed four signals for cycloheptatrienyl carbons suggesting the presence of a pseudo-mirror-plane. Two of four cycloheptatrienyl carbon signals appeared at the high field region due to coordination; i.e. δ = 66.6 ppm [C1], 60.3 ppm [C2], δ = 127.1 ppm [C3 or C4], 126.1 ppm [C3 or C4], where each 13C{1H} signal was assigned with aid of HSQC analyses (cf. δ = 155.1 ppm for the carbons of free tropylium salt). Such 1H and 13C{1H} NMR signal patterns are consistent with those expected for a mononuclear η3-cycloheptatrienyl complex [Pt(η3-C7H7)(CD3CN)2][BF4] (2-CD33CN) (Scheme 5A), and the observed dynamic behavior is probably due to the slipping of the η3-bound platinum center on the cycloheptatrienyl ring.
1 relative intensity (δ = 5.87 ppm [H3/H4 overlap], 5.58 ppm [H2], 3.27 ppm [H1]) (Fig. 6). The 13C{1H} NMR spectra at −80 °C showed four signals for cycloheptatrienyl carbons suggesting the presence of a pseudo-mirror-plane. Two of four cycloheptatrienyl carbon signals appeared at the high field region due to coordination; i.e. δ = 66.6 ppm [C1], 60.3 ppm [C2], δ = 127.1 ppm [C3 or C4], 126.1 ppm [C3 or C4], where each 13C{1H} signal was assigned with aid of HSQC analyses (cf. δ = 155.1 ppm for the carbons of free tropylium salt). Such 1H and 13C{1H} NMR signal patterns are consistent with those expected for a mononuclear η3-cycloheptatrienyl complex [Pt(η3-C7H7)(CD3CN)2][BF4] (2-CD33CN) (Scheme 5A), and the observed dynamic behavior is probably due to the slipping of the η3-bound platinum center on the cycloheptatrienyl ring.
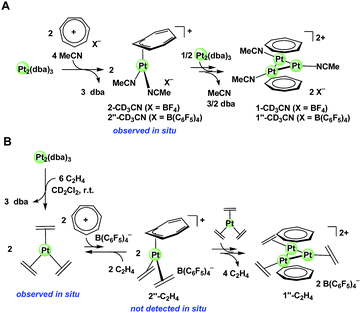 | ||
| Scheme 5 (A) The formation of the mononuclear intermediates and subsequent conversion to the trinuclear sandwich complexes in the presence of acetonitrile. (B) The proposed equilibrium formation of the mononuclear intermediate and subsequent conversion to the trinuclear sandwich complex in the presence of ethylene. | ||
![1H NMR spectra recorded after mixing Pt2(dba)3 and [C7H7][BF4] in CD2Cl2/CD3CN (v/v = 2/1), where a large amount of Pt2(dba)3 remained undissolved. The signals (X) are attributed to impurities.](/image/article/2011/SC/c0sc00269k/c0sc00269k-f6.gif) | ||
| Fig. 6 1H NMR spectra recorded after mixing Pt2(dba)3 and [C7H7][BF4] in CD2Cl2/CD3CN (v/v = 2/1), where a large amount of Pt2(dba)3 remained undissolved. The signals (X) are attributed to impurities. | ||
In the presence of ethylene (1 atm) instead of acetonitrile, the mononuclear intermediate [Pt(η3-C7H7)(C2H4)2][B(C6F5)4] (2′′-C22H44) was not detected by 1H NMR monitoring experiments of the reaction of Pt2(dba)3 with [C7H7][B(C6F5)4]§ (the molar ratio [Pt]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C7H7]+ = 3
[C7H7]+ = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 25 °C in CD2Cl2, while [Pt3(μ3-C7H7)2(C2H4)3][B(C6F5)4]2 (1′′-C22H44) was formed gradually (64% NMR yield after 1 day). In this case, generation of Pt(C2H4)39c–f was observed at the initial stage of the reaction as the major soluble Pt0 species.¶ Variable temperature analyses showed that Pt(C2H4)3, free [C7H7]+, and free ethylene are involved in a dynamic process. Thus, the 1H NMR spectra at −90 °C showed a broad ethylene proton signal of Pt(C2H4)3 with 195Pt satellite peaks at δ = 3.12 ppm, and the sharp singlet signals of free [C7H7]+ and free ethylene, respectively. Raising the temperature to 25 °C resulted in significant broadening of the signals of Pt(C2H4)3 and free [C7H7]+, and slight broadening of that of free ethylene. This temperature dependent NMR behavior can be explained by the equilibrium formation of the mononuclear intermediate 2′′-C22H44 where the equilibrium lies almost on {Pt(C2H4)3 + [C7H7]+} (Scheme 5B).||
2) at 25 °C in CD2Cl2, while [Pt3(μ3-C7H7)2(C2H4)3][B(C6F5)4]2 (1′′-C22H44) was formed gradually (64% NMR yield after 1 day). In this case, generation of Pt(C2H4)39c–f was observed at the initial stage of the reaction as the major soluble Pt0 species.¶ Variable temperature analyses showed that Pt(C2H4)3, free [C7H7]+, and free ethylene are involved in a dynamic process. Thus, the 1H NMR spectra at −90 °C showed a broad ethylene proton signal of Pt(C2H4)3 with 195Pt satellite peaks at δ = 3.12 ppm, and the sharp singlet signals of free [C7H7]+ and free ethylene, respectively. Raising the temperature to 25 °C resulted in significant broadening of the signals of Pt(C2H4)3 and free [C7H7]+, and slight broadening of that of free ethylene. This temperature dependent NMR behavior can be explained by the equilibrium formation of the mononuclear intermediate 2′′-C22H44 where the equilibrium lies almost on {Pt(C2H4)3 + [C7H7]+} (Scheme 5B).||
As mentioned, no reaction took place when Pt2(dba)3 was treated with [C7H7]+ without adding any other ligand. Thus, addition of acetonitrile or ethylene accelerates the formation of the mononuclear intermediate from Pt2(dba)3 and tropylium salt. The DBA ligand, which is an electron-deficient olefin, poorly stabilizes the mononuclear cycloheptatrienyl PtII intermediate by coordination. In contrast, acetonitrile acts as a much better donor ligand than DBA for a PtII center, and thus replacement of DBA ligands with acetonitrile ligands might result in greater stabilization of the mononuclear PtII intermediate. Ethylene acts as the stabilizer for either Pt0 or PtII species.
For the efficient construction of the triplatinum sandwich framework, it is needed that the mononuclear intermediate is transformed facilely. Unfortunately, the intermediates of the transformation from the mononuclear intermediate to the triplatinum sandwich complexes have not been observed. However, dissociation of the ligand from the Pt center is likely to be involved in the transformation of the mononuclear intermediate, in view of the fact that PPh3, pyridine, and COD ligands which coordinate to a PtII center more rigidly than acetonitrile and ethylene ligands retard the transformation of the mononuclear intermediate. The possible pathways are shown in Scheme 6. In path A, the ligand dissociation from the mononuclear PtII intermediate yields a PtII dimer, which then absorbs a Pt0 species to afford the Pt3 sandwich complex. In path B, addition of a Pt0 species to the mononuclear intermediate yields a dinuclear half-sandwich intermediate, which then reacts with the mononuclear intermediate via ligand dissociation to afford the Pt3 sandwich complex. It should be mentioned that the steric factor of the ligands may also be important in the formation of the PtII dimer in path A, or in the formation of the dinuclear half-sandwich intermediate in which the Pt moieties are located synfacially in path B.
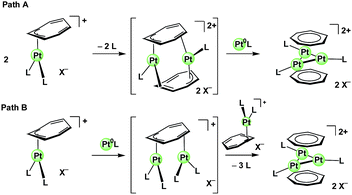 | ||
| Scheme 6 Possible pathways for the transformation of the mononuclear PtII intermediate to the triplatinum sandwich complexes. | ||
Conclusions
In summary, a series of triangular triplatinum sandwich complexes of cycloheptatrienyl were isolated, and the tris-ethylene- and bis-ethylene-aryl Pt3 sandwich complexes were structurally characterized by X-ray crystallographic analyses. The successful synthesis of these triangular triplatinum sandwich complexes from Pt2(dba)3 and a tropylium salt is highly dependent on the nature of the added ligands. Such a ligand effect is rationalized by a mechanism involving a Pt intermediate in a higher oxidation state. The mononuclear PtII adduct of cycloheptatrienyl [Pt(η3-C7H7)(MeCN)2]+ was observed in situ by NMR spectroscopy during the formation of [Pt3(μ3-C7H7)2(MeCN)3]2+. This initially formed intermediate is proven to be the key in the efficient construction of the triangular triplatinum sandwich framework. Acetonitrile and ethylene facilitate generation of the mononuclear PtII intermediate and do not retard the transformation of the intermediate to the corresponding trinuclear sandwich complexes bearing acetonitrile and ethylene. In contrast, addition of more strongly coordinating ligands such as PPh3, pyridine, or 1,5-cyclooctadiene facilitate the formation of the mononuclear PtII adduct but retard the following Pt assembling and sandwiching steps due to the difficulty of the dissociation of the ligands in the mononuclear PtII intermediate.The present results bring a sandwich motif to the molecular design of the triplatinum clusters, one of the classic metal clusters.8,13 The triplatinum sandwich skeletons are highly robust even in the presence of excess coordinative substrates, as found for the related Pd3 sandwich frameworks of cycloheptatrienyl.1,4,14 In contrast, the sandwich framework of the metallocene derivatives of mono- and dinuclear platinum complexes [e.g.[Pt(C5Me5)2]2+ and Pt(allyl)(C5H5)] are usually labile in the presence of coordinative substrates.15 The great stability of the trinuclear sandwich framework of Pt and Pd1 may change the commonly accepted concept that organometallic sandwich complexes of Group 10 metals are not stable in the presence of excess coordinative substrates.16 We expect that the triangular triplatinum sandwich framework of cycloheptatrienyl17 may become a new, versatile molecular design for platinum complexes and catalysts.
Acknowledgements
This work was supported by the Japan Science and Technology Agency and the Ministry of Education, Science, Sports, and Technology, Japan. Thanks are also given to the Analytical Center, Faculty of Engineering, Osaka University for the use of the NMR facilities.Notes and references
- T. Murahashi, M. Fujimoto, M. Oka, Y. Hashimoto, T. Uemura, Y. Tatsumi, Y. Nakao, A. Ikeda, S. Sakaki and H. Kurosawa, Science, 2006, 313, 1104 CrossRef CAS.
- T. Murahashi, N. Kato, T. Uemura and H. Kurosawa, Angew. Chem., Int. Ed., 2007, 46, 3509 CrossRef CAS.
- T. Murahashi, M. Fujimoto, Y. Kawabara, R. Inoue, S. Ogoshi and H. Kurosawa, Angew. Chem., Int. Ed., 2007, 46, 5440 CrossRef CAS.
- T. Murahashi, Y. Hashimoto, K. Chiyoda, M. Fujimoto, T. Uemura, R. Inoue, S. Ogoshi and H. Kurosawa, J. Am. Chem. Soc., 2008, 130, 8586 CrossRef CAS.
- T. Murahashi, R. Inoue, K. Usui and S. Ogoshi, J. Am. Chem. Soc., 2009, 131, 9888 CrossRef CAS.
- T. Murahashi, T. Nagai, T. Okuno, T. Matsutani and H. Kurosawa, Chem. Commun., 2000, 1689 RSC.
- (a) K. Moseley and P. M. Maitlis, J. Chem. Soc., Dalton Trans., 1974, 169 RSC; (b) H. Tanaka and H. Kawazura, Bull. Chem. Soc. Jpn., 1979, 52, 2815 CAS.
- (a) G. Booth, J. Chatt and P. Chini, J. Chem. Soc., Chem. Commun., 1965, 639 RSC; (b) G. Booth and J. Chatt, J. Chem. Soc. A, 1969, 2131 RSC; (c) J. Chatt and P. Chini, J. Chem. Soc. A, 1970, 1538 RSC; (d) A. Albinati, Inorg. Chim. Acta, 1977, 22, L31 CrossRef CAS . For selected reviews on triangular triplatinum clusters; (e) A. D. Burrows and D. M. P. Mingos, Coord. Chem. Rev., 1996, 154, 19 CrossRef CAS; (f) D. Imhof and L. M. Venanzi, Chem. Soc. Rev., 1994, 23, 185 RSC; (g) R. J. Puddephatt, Lj. Manojlovic-Muir and K. W. Muir, Polyhedron, 1990, 9, 2767 CrossRef CAS; (h) D. M. P. Mingos and R. W. M. Wardle, Transition Met. Chem., 1985, 10, 441 CAS; (i) P. Chini, J. Organomet. Chem., 1980, 200, 37 CrossRef CAS.
- For Pt(η2-C2H4)(PPh3)2: (a) C. D. Cook and G. S. Jauhal, Inorg. Nucl. Chem. Lett., 1967, 3, 31 CrossRef CAS; (b) P.-T. Cheng and S. C. Nyburg, Can. J. Chem., 1972, 50, 912 CAS For [Pt(C2H4)3]:; (c) M. Green, J. A. K. Howard, J. L. Spencer and F. G. A. Stone, J. Chem. Soc., Chem. Commun., 1975, 3 RSC; (d) M. Green, J. A. K. Howard, J. L. Spencer and F. G. A. Stone, J. Chem. Soc., Chem. Commun., 1975, 449 RSC; (e) M. Green, J. A. K. Howard, J. L. Spencer and F. G. A. Stone, J. Chem. Soc., Dalton Trans., 1977, 271 RSC; (f) J. A. K. Howard, J. L. Spencer and S. A. Mason, Proc. R. Soc. London, Ser. A, 1983, 386, 145 CAS . For a review on the first PtII ethylene complex (the Zeise's salt); (g) D. Seyferth, Organometallics, 2001, 20, 2 CrossRef CAS and references therein. For structural studies of the Zeise's complexes; (h) R. A. Love, T. F. Koetzle, G. J. B. Williams, L. C. Andrews and R. Bau, Inorg. Chem., 1975, 14, 2653 CrossRef CAS; (i) S. Otto, A. Roodt and L. I. Elding, Inorg. Chem. Commun., 2006, 9, 764 CrossRef CAS; (j) N. M. Boag and M. S. Ravetz, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m3103 CrossRef.
- (a) R. Ros, G. Facchin, A. Tassan, R. Roulet, G. Laurenczy and F. Lukacs, J. Cluster Sci., 2001, 12, 99 CrossRef CAS; (b) P. Leoni, F. Marchetti, M. Pasquali, L. Marchetti and A. Albinati, Organometallics, 2002, 21, 2176 CrossRef CAS; (c) P. Leoni, F. Marchetti, L. Marchetti and V. Passarelli, Chem. Commun., 2004, 2346 RSC.
- For example, 1JPt–P = 4919 Hz, 2JPt–P = 459 Hz, and 3JP–P = 54 Hz for Pt3(μ-CO)3(PPh3)3; 1JPt–P = 4412 Hz, 2JPt–P = 430 Hz, and 3JP–P = 58 Hz for Pt3(μ-CO)3(PCy3)3. (a) A. Moor, P. S. Pregosin and L. M. Venanzi, Inorg. Chim. Acta, 1981, 48, 153 CrossRef CAS; (b) M. Green, R. M. Mills, G. N. Pain, F. G. A. Stone and P. Woodward, J. Chem. Soc., Dalton Trans., 1982, 1309 RSC.
- M. Green, D. M. Grove, J. L. Spencer and F. G. A. Stone, J. Chem. Soc., Dalton Trans., 1977, 2228 RSC.
- For selected recent examples of triangular triplatinum clusters: (a) R. Bender, P. Braunstein, A. Dedieu, P. D. Ellis, B. Huggins, P. D. Harvey, E. Sappa and A. Tiripicchio, Inorg. Chem., 1996, 35, 1223 CrossRef CAS; (b) K. Osakada, M. Tanabe and T. Tanase, Angew. Chem., Int. Ed., 2000, 39, 4053 CrossRef CAS; (c) L. R. Falvello, J. Fornies, C. Fortuno, F. Duran and A. Martin, Organometallics, 2002, 21, 2226 CrossRef CAS; (d) R. Bender, C. Okio, R. Welter and P. Braunstein, Dalton Trans., 2009, 4901 RSC; (e) C. Cavazza, F. F. de Biani, T. Funaioli, P. Leoni, F. Marchetti, L. Marchetti and P. Zanello, Inorg. Chem., 2009, 48, 1385 CrossRef CAS.
- (a) F. L. Mulligan, D. C. Babbini, I. R. Davis, S. K. Hurst and G. S. Nichol, Inorg. Chem., 2009, 48, 2708 CrossRef CAS; (b) D. C. Babbini, F. L. Mulligan, H. R. Schulhauser, T. C. Sweigart, G. S. Nichol and S. K. Hurst, Inorg. Chem., 2010, 49, 4307 CrossRef CAS.
- (a) B. L. Shaw and N. Sheppard, Chem. & Ind., 1961, 517 Search PubMed; (b) B. E. Mann, B. L. Shaw and G. Shaw, J. Chem. Soc. A, 1971, 3536 RSC; (c) H. Werner and A. Kuhn, Angew. Chem., Int. Ed. Engl., 1977, 16, 412 CrossRef; (d) G. E. Herberich, U. Englert and F. Marken, J. Chem. Soc., Dalton Trans., 1993, 1979 RSC; (e) O. V. Gusev, L. N. Morozova, T. A. Peganova, M. G. Peterleitner, S. M. Peregudova, L. I. Denisovich, P. V. Petroskii, Y. F. Oprunenko and N. A. Ustynyuk, J. Organomet. Chem., 1995, 493, 181 CrossRef CAS; (f) Y. T. Struchkov, M. Y. Antipin, K. A. Lyssenko, O. V. Gusev, T. A. Peganova and N. A. Ustynyuk, J. Organomet. Chem., 1997, 536–537, 281 CrossRef.
- (a) B. L. Shaw, Proc. Chem. Soc., 1960, 247 Search PubMed; (b) E. O. Fischer and H. Werner, Tetrahedron Lett., 1961, 2, 17 CrossRef; (c) E. O. Fischer and G. Burger, Chem. Ber., 1961, 94, 2409 CrossRef CAS; (d) G. Wilke and B. Bogdanovic, Angew. Chem., 1961, 73(23), 756 CrossRef CAS; (e) H. Werner, A. Kuhn, D. J. Tune, C. Kruger, D. J. Brauer, J. C. Sekutowski and Y.-H. Tsay, Chem. Ber., 1977, 110, 1763 CrossRef CAS; (f) B. Henc, P. W. Jolly, R. Salz, S. Stobbe, G. Wilke, R. Benn, R. Mynott, K. Seevogel, R. Goddard and C. Kruger, J. Organomet. Chem., 1980, 191, 449 CrossRef CAS.
- For a review of cycloheptatrienyl transition metal complexes: M. L. H. Green and D. K. P. Ng, Chem. Rev., 1995, 95, 439 Search PubMed.
- (a) W. V. Konze, B. L. Scott and G. J. Kubas, Chem. Commun., 1999, 1807 RSC; (b) J. S. Owen, J. A. Labinger and J. E. Bercaw, J. Am. Chem. Soc., 2006, 128, 2005 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental, spectroscopic and crystallographic details. CCDC reference numbers 773671–773675. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00269k/ |
| ‡ The B(C6F5)4 salt of tropylium was used because of its improved solubility compared to the BF4 salt. |
| § When the B(ArF)4 (ArF = 3,5-(CF3)2(C6H3)) salt of tropylium was used, formation of the mononuclear adduct [Pd(C7H7)(CD3CN)2][B(ArF)4] (2′-CH33CN) was observed. However, this reaction ultimately resulted in at least two products showing a cycloheptatrienyl signal at 4.16 ppm and 3.92 ppm, respectively. The products have not been identified, but one of the unidentified products is probably an arylated product [Pd3(C7H7)2(CD3CN)2(ArF)][B(ArF)4]. We confirmed that the reaction of Pt2(dba)3 with [C7H7][B(ArF)4] in the presence of ethylene at 25 °C in CD2Cl2 unexpectedly gave the cationic triplatinum sandwich complex [Pt3(μ3-C7H7)2(ArF)(C2H4)2][B(ArF)4] bearing a 3,5-di-trifluoromethylphenyl ligand, where only a trace amount of tris-ethylene complex 1′-C22H44 was formed. 1′-C22H44 was stable in CD2Cl2. The structure of [Pt3(μ3-C7H7)2(ArF)(C2H4)2][B(ArF)4] was determined by X-ray crystallographic analysis (See Supporting Information†). It was reported that the B(ArF)4 anion reacts with a mononuclear cationic Pt(II) center bearing a weakly coordinated solvent ligand to afford the transmetallation product.18 |
| ¶ Pt(C2H4)3 was generated by the reaction of Pt2(dba)3 and ethylene in CD2Cl2 at 25 °C (1H NMR, δ = 3.21 ppm, JPt-H = 57 Hz). |
| || Attempts to detect the mononuclear adduct by treatment of Pt2(dba)3 with excess [C7H7][BF4] in the presence of ethylene in CD2Cl2/CD3NO2 (v/v = 2/1) failed. |
| This journal is © The Royal Society of Chemistry 2011 |
