Synthesis of novel β-aminocyclobutanecarboxylic acid derivatives by a solvent-free aza–Michael addition and subsequent ring closure†‡
Abstract
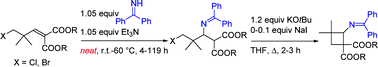
Novel β-aminocyclobutanecarboxylic acid derivatives were prepared via a sequential
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...