A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application†
Yingying
Lv
a,
Fan
Zhang
*a,
Yuqian
Dou
b,
Yunpu
Zhai
a,
Jinxiu
Wang
a,
Haijing
Liu
a,
Yongyao
Xia
a,
Bo
Tu
a and
Dongyuan
Zhao
*a
aDepartment of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Laboratory of Advanced Materials, Fudan University, Shanghai, 200433, P. R. China. E-mail: zhang_fan@fudan.edu.cn; dyzhao@fudan.edu.cn; Fax: +86-21-51630307; Tel: +86-21-51630205
bDepartment of Chemistry, Northeast University, Shenyang, 110819, P. R. China
First published on 11th October 2011
Abstract
Activation of ordered mesoporous carbon orientates the development and application of new carbonaceous supercapacitor materials with high energy density and power density. Ordered mesoporous carbons FDU-15 are synthesized in large scale via a soft template method through evaporation induced self-assembly of mesostructure on the sacrificed polyurethane foam. Common activating agent potassium hydroxide (KOH) is utilized to improve the surface area and tailor the pore texture of the ordered mesoporous carbon by adjusting KOH/carbon mass ratio as well as activation time. At low KOH/carbon ratio, the generated micropores increase in volume and either connect to other micropores or eventually become mesopores. At high KOH/carbon ratio, an excess amount of micropores would be generated. Meanwhile, the continuous shrinkage of carbon framework is carried through as prolonged time at high activation temperature. Competition between KOH etching and shrinkage of mesopores is existed during the activation. The latter obviously preponderates over the former at low KOH/carbon ratio, which is reversed at high KOH/carbon ratio. Thus, an optimized micro-mesostructure is achieved under certain activation conditions: maintained ordered mesostructure, suitable microporosity, high surface area (1410 m2 g−1) and large pore volume (0.73 cm3 g−1). The activated sample exhibits improved electrochemical behavior with a gravimetric capacitance of 200 F/g, excellent rate performance and good cycling stability with capacitance retention of ∼98% over 300 cycles.
1. Introduction
A developing modern society brings an unavoidable energy crisis, which necessitates innovative research in clean energy technologies such as charge energy storage for mobile devices or next-generation electric vehicles. The power requirements of fully electric vehicles demand highly efficient current charge/discharge capabilities and high power density storage. Such features are lacking in currently used batteries. This need has steered active research into alternative approaches such as supercapacitors which have high power density and long life cycles (>100 times of battery life). Supercapacitors, which are also called the electrical double layer capacitors (EDLC), store energy by charge separation in the electrode/electrolyte interface.1–4 Currently, in comparison with batteries, supercapacitors with high-surface-area carbon materials as electrodes exhibit a lower energy density (<10 Wh/kg) in aqueous electrolytes. To date, carbon electrode materials have played important roles in the future supercapacitors due to the good electric conductivity, long cycle stability and stable physicochemical properties. Activated carbon has earned its status in EDLC capacitor electrode materials due to its cost effectiveness, largely owing to the large specific surface area that has the ability to accumulate a large number of charges. However, the micropores (<2 nm) of activated carbon are difficult to be completely wet with electrolyte. Hence, an undesired decrease in capacitance is evident at high current density due to the resistance to the diffusion and transport of electrolyte ions in inner-pores.5,6 Therefore, high rate performance could not be realized, which is very important for high power supercapacitors. Recent studies have proved that the carbon-based micro/mesoporous materials (with pore width of about 0.7–3 nm allowing for solvated electrolyte ion) can function as high energy storage EDLC.7 Furthermore, 2-D hexagonal mesoporous carbon is more favorable for ion diffusion than that with isolated 3-D cubic and disordered wormlike pore characteristics, which can deliver better capacitance retention and lower impedance to electrode kinetic processes.8 Consequently, mesoporous carbons with ordered mesostructure, high surface area, and suitable pore size have been proposed as promising supercapacitor electrode materials with high power density.9Our group has successfully developed a soft template method to synthesize highly ordered 2-D hexagonal mesoporous carbon FDU-15 by using amphiphilic triblock copolymers (PEO-PPO-PEO) as template phenolic resin as carbon precursor via a solvent evaporation induced self-assembly method (EISA) followed by a thermopolymerization process.10 Recently, a simple strategy has been reported for the synthesis of ordered mesoporous carbon by using polyurethane foam scaffolds as decomposable substrates for the EISA step.11 After carbonization, the substrates (polyurethane foam) are burned out, and ordered mesoporous carbon is obtained in large scale, which makes it possible for commercial use. However, the specific surface area of the obtained ordered mesoporous carbon FDU-15 is relatively low (660 m2 g−1), which is disadvantageous for charge storage. In order to obtain ordered mesoporous carbon with desirable electrochemical capacitive performance, further research into subsequent activation is required. Industrially, KOH activation is a well-established method that can be used for generating micropores in activated carbon.12,18–20 Up to now, some studies have been reported for the KOH activation of mesoporous carbon, and show the improvement of specific surface area.13–15KOH activated mesoporous carbon (KOH/carbon ratio of 6.0, activation temperature of 800 °C and activation time of 1 h) with high surface area of 1940 m2 g−1 at the expense of the mesostructural order, shows a improved capacitance of 188 F/g at a low scan rate of 1 mV s−1 in 0.1 M NaCl electrolyte.16 Under the conditions of KOH/carbon ratio of 4.0, activation temperature of 800 °C and activation time of 1 h, ordered mesostructure of the activated mesoporous carbon is retained. A capacitance of 180 F/g is obtained with a frequency of 1 Hz in 30% KOH electrolyte.17 Increasing the activation temperature up to 1000 °C, the activated mesoporous carbon demonstrates higher surface area of 2060 m2 g−1 but a relatively low capacitance of 60 F/g.17 Although increasing the KOH/carbon ratio or activation temperature can be utilized to improve the surface area of mesoporous carbon, it is unfavorable from both economical and environmental perspectives. Hence, it is quite important to systematically study the KOH activation on mesostucture or pore evolution and eventual effect on the electrochemical performance of soft template synthesized ordered mesoporous carbon. Moreover, given their great potential and ease of industry-scale production, it is desirable to generate new pores in such novel carbon materials for electro-chemical applications.
In this paper, the KOH activation of the ordered mesoporous carbon is systematically investigated by adjusting a series of activation conditions at various KOH/carbon mass ratios and activation time. It is found that FDU-15 shows excellent mesostructural stability even under severe activation conditions. The effects of KOH activation on the mesostructure, pore size and surface area of FDU-15 are conveniently studied in detail. Meanwhile, a large number of micropores or mesopores are generated, and their contents and properties are determined. Moreover, the activated mesoporous carbon can be used as high performance supercapacitor electrode material with high energy density and power density.
2. Experimental
2.1. Chemicals
Triblock poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) copolymer Pluronic F127 (Mw = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, EO106-PO70-EO106) was purchased from Acros Corp. Polyether polyol-based polyurethane (PU) foam, an industrial product with a porosity of 50 ppi (pores per linear inch), was purchased from Shanghai Changda Foam Co. Ltd. The density of the PU foam was 0.02 g cm−3. Other chemicals were purchased from Shanghai Chemical Company. All chemicals were used as received without any further purification. Millipore water was used in all experiments.
600, EO106-PO70-EO106) was purchased from Acros Corp. Polyether polyol-based polyurethane (PU) foam, an industrial product with a porosity of 50 ppi (pores per linear inch), was purchased from Shanghai Changda Foam Co. Ltd. The density of the PU foam was 0.02 g cm−3. Other chemicals were purchased from Shanghai Chemical Company. All chemicals were used as received without any further purification. Millipore water was used in all experiments.
2.2 Synthesis of ordered mesoporous carbon
Ordered mesoporous carbon FDU-15 with 2-D hexagonal structure was synthesized by using phenol/formaldehyde resol as carbon precursor, triblock copolymer F127 as template for the mesostructure and PU foam as sacrificial scaffold. Firstly, 1.0 g of F127 was dissolved in 20.0 g of ethanol. Then 5.0 g of the resol precursor in ethanol solution containing 0.61 g of phenol and 0.39 g of formaldehyde was added under stirring.10 The resulting homogeneous solution was uniformly coated on the 1.0 g of the PU foam. After the ethanol was evaporated completely at room temperature, samples were stabilized at 150 °C for 24 h. Thermal treatment of the resulting carbon was performed in the tube furnace under nitrogen atmosphere with a flow rate of 100 cm3 min−1 for carbonizing at 700 °C for 3 h. The resulted ordered mesoporous carbon was denoted as FDU-15.2.3 Activation of ordered mesoporous carbon
Ordered mesoporous carbon FDU-15 was activated by KOH under various conditions by adjusting the KOH/carbon mass ratio (1.0–6.0) as well as the activation time (45–90 min). For a typical activation, 0.25 g of FDU-15 was impregnated with KOH solution (0.25g in 5 g ethanol), followed by an evaporation step at 60 °C under stirring in nitrogen atmosphere. The activation process was carried out in a tube furnace under flowing nitrogen with a flow rate of 100 cm3 min−1 by heating the sample with a rate of 5 °C min−1 up to 700 °C and holding at this temperature for 90 min. The resulting mixture (after cooled in flowing nitrogen to room temperature) was washed with 1 M HCl solution and copious amounts of millipore water and then dried at 100 °C for 12 h. The activated samples were denoted as KF-x-y, where KF means KOH activated mesoporous carbon FDU-15, x means the KOH/carbon mass ratio, y refers to the activation time.2.4. Characterization
The small-angle X-ray scattering (SAXS) measurements were taken on a Nanostar U small-angle X-ray scattering system (Bruker, Germany) using Cu-Kα radiation (40 kV, 35 mA). The d-spacing values were calculated using the formula d = 2π/q, and the unit cell parameters (a0) were calculated from the formula a0 = 2d10/√3. Nitrogen sorption isotherms were measured on a Micromeritics Tristars 3000 analyzer at 77 K. Before the measurements, the samples were outgassed at 180 °C in vacuum for 6 h. The Brumauer–Emmett–Teller (BET) method was utilized to calculate the specific surface areas. The pore size distribution was derived from the adsorption branches of the isotherms, and the total pore volumes (Vt) were estimated from the adsorbed amount at a relative pressure P/P0 of 0.995. The micropore surface area was calculated by using t-plot method with a relative pressure P/P0 of 0.2–0.5. The micropore volume (Vm) and micropore surface area were calculated by using the V–t plot method. The t values were calculated as a function of the relative pressure using the de Boer equation t/A° = [13.99/(log(P0/P) + 0.034)]1/2. Vm was calculated by using the equation, Vm/cm3 = 0.001547I, where I represents the Y intercept in the V–t plot. Transmission electron microscopy (TEM) experiments were conducted on a JEOL 2011 microscope (Japan) operated at 200 kV. The samples for TEM measurements were suspended in ethanol and supported onto a lacey carbon film on a copper grid.2.5. Electrochemical measurement
To prepare the working electrodes, a mixture of 85 wt% carbon samples, 10 wt% acetylene black, and 5 wt% polytetrafluoroethylene (PTFE) was dispersed in alcohol and pressed on nickel foam under 20 MPa. Then, the electrodes were dried at 100 °C for 12 h. The typical mass load of the electrode was 5 mg cm−2. The electrochemical performances of the prepared electrodes were characterized by cyclic voltammetry (CV) and charge–discharge tests. 6.0 M KOH solution was used as the electrolyte. The experiments were carried out using a three-electrode cell, in which platinum and Hg/HgO electrodes (in 6.0 M KOH) were used as counter and reference electrodes, respectively. The experiments were performed on an electrochemical analyzer (CHI 760) under ambient conditions.3. Results and discussion
3.1 Mesostructure and pore texture evolution under low KOH/carbon ratios
SAXS pattern (Fig. 1) of the pristine mesoporous carbon FDU-15 reveals three well-resolved scattering peaks which can be indexed as 10, 11 and 20 reflections associated with 2-D hexagonal symmetry (space group of p6m). After KOH activation for 45, 60 and 90 min with KOH/carbon mass ratio of 1.0, all the activated samples present distinct 10 and 11 scattering peaks (Fig. 1A), indicating that the mesostructure regularity can be well retained. When activation time is prolonged from 45 to 90 min, the scattering peaks of activated samples with KOH/carbon ratio of 1.0 become remarkably broad and the intensities of which decrease obviously (Fig. 1A), suggesting the degradation of ordered mesostructure as the increasing activation time (Table 1). Compared with the pristine FDU-15, all the scattering peaks of activated sample with KOH/carbon mass ratio of 1.0 under the activation time of 45 min are slightly shifted to lower q value (Fig. 1). The unit cell parameter (a0) is slightly increased from 10.2 to 10.7 nm, implying a 4.9% framework expansion caused by the etching of mesopore walls by KOH (Table 1). After being treated up to 90 min, the scattering peaks shift to higher q, and the unit cell parameter (a0) is calculated to be 9.8 nm, reflecting minor framework shrinkage of 3.9%.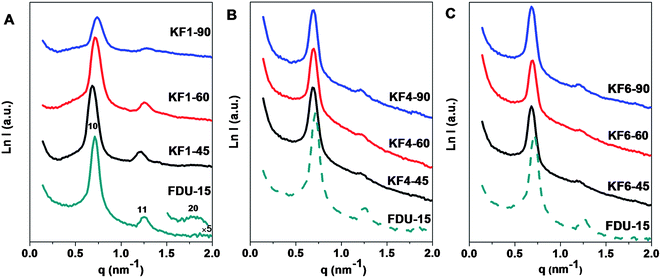 | ||
| Fig. 1 SAXS patterns of FDU-15 and activated samples with KOH/carbon ratio of 1.0 (A), 4.0 (B), 6.0 (C) for 45, 60, 90 min, respectively. | ||
| Sample | Unit cell size | BET surface area | Pore volume | Micropore surface area | Microporosity (surface area) | Micropore volume |
|---|---|---|---|---|---|---|
| (nm) | (cm2 g−1) | (cm3 g−1) | (cm2 g−1) | (%) | (cm3 g−1) | |
| FDU-15 | 10.2 | 660 | 0.44 | 180 | 27 | 0.07 |
| KF1-45 | 10.7 | 930 | 0.49 | 590 | 63 | 0.24 |
| KF1-60 | 10.2 | 1030 | 0.52 | 660 | 64 | 0.27 |
| KF1-90 | 9.8 | 1410 | 0.73 | 890 | 63 | 0.38 |
| KF4-45 | 10.5 | 1150 | 0.56 | 830 | 72 | 0.34 |
| KF4-60 | 10.3 | 1310 | 0.62 | 1030 | 79 | 0.43 |
| KF4-90 | 10.3 | 1240 | 0.59 | 970 | 78 | 0.40 |
| KF6-45 | 10.7 | 1280 | 0.59 | 990 | 77 | 0.41 |
| KF6-60 | 10.6 | 1400 | 0.69 | 1020 | 73 | 0.42 |
| KF6-90 | 10.6 | 1200 | 0.56 | 960 | 80 | 0.40 |
A more detailed structural characterization is revealed by TEM images, the pristine FDU-15 shows highly ordered stripe-like (from [11] direction) and hexagonally arranged (from [01] direction) images (Fig. 2A, B). After KOH treatment with KOH/carbon ratio of 1.0 for 90 min, the activated sample exhibits 2-D hexagonal regularity, suggesting good framework stability under a long activation time (Fig. 2C, D). Estimated from TEM images, the cell parameter decreases from ∼10.4 to 10.1 nm for the pristine FDU-15 and activated sample KF1–90, respectively, which are accordance with the shrinkage degree calculated from SAXS data. Compared with that of pristine FDU-15 (∼5 nm), the mesopore size of activated sample KF1–90 shrinks to ∼3–4 nm (Fig. 2D), which is in agreement with the degree of shrinkage of the framework. It should be noted that, the formation of KOH generated micro/mesopores in the wall is obvious in the activated sample under the conditions of KOH/carbon ratio of 1.0 and long activation time of 90 min (Fig. 2C).
![TEM images of ordered mesoporous carbon FDU-15 (A, B) and activated sample KF1–90 (C, D), KF6–90 (E, F), viewed from [11] (A, C, E) and [01] (B, D, F) directions. The insets are the corresponding FFT diffractograms.](/image/article/2012/JM/c1jm12742j/c1jm12742j-f2.gif) | ||
| Fig. 2 TEM images of ordered mesoporous carbon FDU-15 (A, B) and activated sample KF1–90 (C, D), KF6–90 (E, F), viewed from [11] (A, C, E) and [01] (B, D, F) directions. The insets are the corresponding FFT diffractograms. | ||
Nitrogen sorption isotherm (Fig. 3A) of the pristine FDU-15 is a representative type-IV curve for the typical H1 hysteresis loop (parallel and nearly vertical branches in the mesopore range) at the evident capillary condensation steps at P/P0 = 0.4–0.6, which are typical features of uniform mesopores. The BET surface area and total pore volume are calculated to be 660 m2 g−1 and 0.44 cm3 g−1, respectively. The pore size distribution (Fig. 3B) calculated from the adsorption branch by NLDFT (Nonlocal Density Functional Theory) method reveals bimodal pores with an average mesopore of ∼5 nm and a micropore of ∼1.5 nm, respectively. After the KOH activation treatment, all the activated samples with KOH/carbon ratio of 1.0 exhibit similar type-IV curves with H1 hysteresis loops at the same P/P0 range as that of the pristine sample FDU-15 (Fig. 3A), revealing that ordered mesostructure can be maintained under the applied activation conditions. However, the surface area and pore volume of all activated samples are larger than that of pristine FDU-15 (Fig. 3A). The surface area of KF1 samples increases from 930 to 1410 m2 g−1 as activation time is prolonged from 45 to 90 min (Table 1). Compared with the intrinsic microporosity of the pristine FDU-15 (27%), the microporosities of KF1 samples (with KOH/carbon ratio of 1) are dramatically increased to ∼63% (Table 1). The condensation steps become wider and the adsorption amounts obviously increase (Fig. 3A), suggesting that mesopore distribution becomes wider and pore volumes increase greatly (Fig. 3B). Remarkably, the activated sample with KOH/carbon ratio of 1.0 for 90 min shows a wider mesopore size distributed between 1.5–4 nm, and a high pore volume of 0.73 cm3 g−1 contributed mostly by micropores (Fig. 3B), which is coincident with TEM images (Fig. 2B).
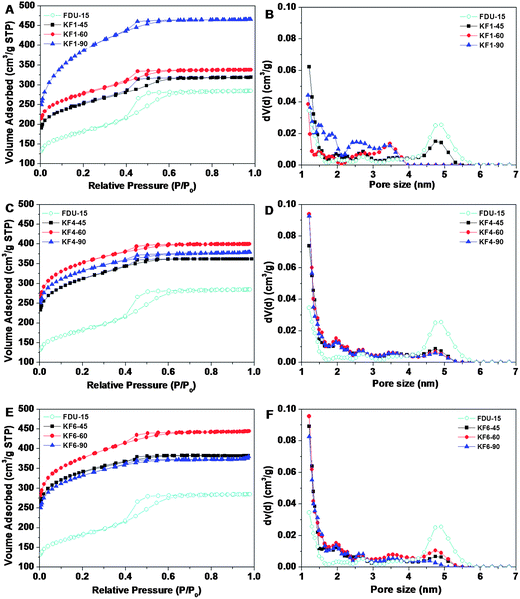 | ||
| Fig. 3 N2 sorption isotherms and pore size distribution of ordered mesoporous carbon FDU-15 and activated samples with KOH/carbon ratio of 1.0 (A, B), 4.0 (C, D), 6.0 (E, F) for 45, 60, 90 min, respectively. | ||
At low KOH/carbon ratio, the KOH generated micropores increase in volume and lead to the degradation of mesostrucutre. The etching action as well as continuous shrinkage of pore wall occurred in KOH activation, leads to expansion and shrinkage of framework, respectively. The small mesopores in the wall caused by activation with low KOH/carbon ratio under long activation time, imply that KOH is homogeneously distributed inside the pore walls on nanoscale. The results show that the activated sample obtained with a KOH/carbon ratio of 1.0 under long activation time of 90 min possesses the highest BET surface area of 1410 m2 g−1 and total pore volume of 0.73 cm3 g−1, respectively, indicating the effectiveness of KOH in developing new pores in mesoporous carbon skeleton.
3.2 Mesostructure and pore texture evolution under high KOH/carbon ratios
As the KOH/carbon ratio increases from 1.0 to 6.0, a gradual decrease in SAXS peak intensity of activated sample with activation time of 45 min occurs (Fig. 1A–C). Moreover, the 10 and 11 scattering peak can be maintained even if the activation time is up to 90 min with high KOH/carbon ratio of 6.0, suggesting excellent framework stability of mesoporous carbon FDU-15 prepared by soft template method. The scattering peak of KF6 samples with different activation time show tiny shift of the all scattering peak to high q value (Fig. 1). The unit cell parameter (a0) increase to 10.7–10.6 nm after activation, implying a 3.9% framework expansion (Table 1). The remarkable framework changes under different activation time are not observable, showing that the shrinkage degree of framework is lower than the etching degree at high KOH/carbon ratio.The TEM images of the activated sample with high KOH/carbon ratio of 6.0 viewed from [11] (Fig. 2E) and [01] (Fig. 2F) directions, respectively, further confirm the well preserved 2-D hexagonal mesostructure. The cell parameter of the activated sample KF6–90 under long activation time of 90 min is estimated to be 10.3 ± 0.2 nm, in good agreement with that determined from the SAXS data. The mesopore size of KF6–90 is about 4.5 nm, which is indicative of smaller framework shrinkage of activated samples with high KOH/carbon ratio than low KOH/carbon ratio. At high KOH/carbon ratio of 6.0, the formation of overabundant micropores distributed in the carbon framework can be observed (Fig. 2F).
Nitrogen sorption isotherms of activated samples with different KOH/carbon ratio show the similar trends of structure evolution. The representative type-IV isotherms and H1 hysteresis loops are observed under the condition of KOH/carbon ratio between 1.0 and 6.0 with activation time of 45 min (Fig. 3A, C, E), reflecting the maintenance of ordered mesostructure even at severe activation conditions. As the KOH/carbon ratio continuously increases from 1.0 to 6.0, the surface area of activated samples is increased from 930 to 1280 m2 g−1 with activation time of 45 min. The sequence of initial parts of adsorption isotherms (Fig. 3A, C, E) as well as the NLDFT pore size distribution (Fig. 3B, D, F) show gradual increase in the micropore volume as KOH/carbon ratio increases (Table 1). The microporosity is increased to 77% as the KOH/carbon ratio is increased (4.0 or 6.0) (Table 1). It should be noted that, the BET surface area of KF6 samples, increases from 1280 to 1400 m2 g−1 with the increasing activation time from 45 to 60 min, but decreases to 1200 m2 g−1 with activation time of 90 min (Table 1). The KF6–90 samples show a highest microporosity of 80%, suggesting that a large amount of micropores are generated instead of being connected to form small mesopores at severe activation condition.
As KOH/carbon ratio increases, the dramatic increasing of micropores is attributed to a fairly larger extent of enlarging surface area and total pore volume. At high KOH/carbon ratio, the competition between the etching of mesopores and shrinkage of frameworks still exists, the former is more obvious. The etching action at high KOH/carbon ratio is more severe than that at low KOH/carbon ratio, so few small mesopores are generated. If activation time is too long, inner ultra-small micropores without the connecting by mesopores are difficult for N2 adsorption, inducing a decrease of surface area and pore volume.
3.3 Understanding the KOH activation of soft templated mesoporous carbon
The mechanism of KOH activation is generally considered as a reaction between KOH and carbon, 6KOH + 2C → 2K + 2K2CO3 + 3H2.18–20 A slight etching of mesopores can be achieved under short activation time of 45 min (Scheme 1b, d), inducing a tiny increase of cell parameter. At high activation temperature of 700 °C, the carbon framework would continue shrink with prolonged time. Meanwhile, a certain amount of metallic potassium was produced to generate corresponding micropores. At low KOH/carbon ratio, active metallic potassium would congregate in the micropores in the carbon framework via capillary interaction, thus enlarge or coalesce the unconnected micropores to new mesopores at a long activation time (Scheme 1c). The existence of newly generated mesopores would weak the intensity of x-ray diffraction. At high KOH/carbon ratio, more micropores were generated due to severe etching action by large amount of metallic potassium, which lead to the degradation of the ordered mesostructure (Scheme 1d, e). There is a competition between mesopores shrinkage and etching in the KOH activation. The former at low KOH/carbon ratio obviously preponderate over the latter, which is reverse at high KOH/carbon ratio (Scheme 1). Since the size of metallic potassium is smaller than N2, the latter is comparable with ions in KOH aqueous soulution.21 The generated over-abundant micropores are difficult for electrolyte wetting, would be a drawback for supercapacitor performance. In conclusion, the optimized activation condition is found to be at a low KOH/carbon ratio (1.0) as well as long activation time (90 min).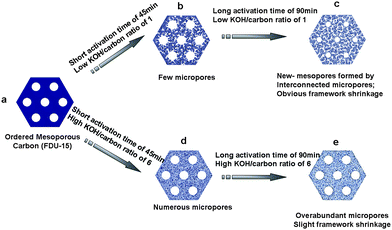 | ||
| Scheme 1 Illustration of KOH activation of ordered mesoporous carbon FDU-15. | ||
3.4 Improvement on electrochemical properties
Typical cyclic voltammograms (CV) at the scan rate of 5 mV s−1 for all the activated carbons in 6.0 M KOH aqueous electrolyte present a quasi-rectangular voltammogram shape, exhibiting typical characteristic of EDLC, indicative of excellent candidates as an electrode materials (Fig. 4A, Fig. S1†). Of special interest is the fact that, with abundant micropores, activated mesoporous carbons still retain good capacitive behavior even at high sweep rates (Fig. S2†). This confirms that the hierarchical pore structure and well connected micropores/mesopores are beneficial for fast ionic transportation within the mesopores and diffusion from mesopores to micropores.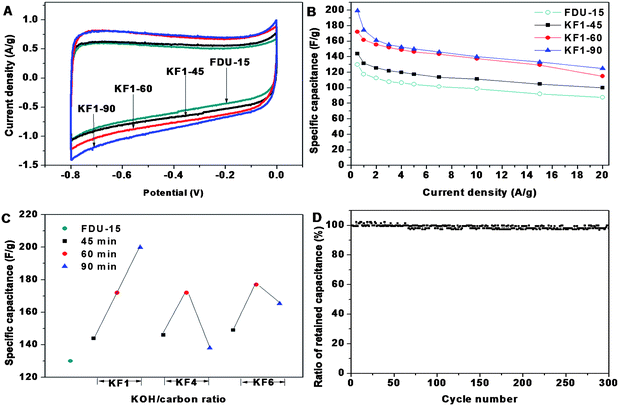 | ||
| Fig. 4 The CV curves at the scan rates of 5 mV s−1 (A) and the dependence of retained capacitance (B) of ordered mesoporous carbon FDU-15 and activated samples with KOH/carbon ratio of 1.0 for 45, 60, 90 min, respectively; specific capacitance of FDU-15 and activated samples versusKOH/carbon ratio and activation time (C); cycle performance of activated sample KF1–90 at the current density of 1.0 A g−1 (D). | ||
Fig. 4B shows the specific capacitance of the pristine FDU-15 and KF1 samples at a wide range of current densities from 0.5 to 20 A/g. The curve of the retained specific capacitance of KF1 samples as the increasing current density is similar to that of pristine FDU-15, showing that the micro-mesostructure still performs with good rate capability. Because the enhanced specific surface area benefits EDLC formation, the specific capacitance of activated samples is larger than that of pristine FDU-15 (130 F/g). The specific capacitance is proportional to their specific surface area at different KOH/carbon ratio (Fig. 4C). The specific capacitances of KF1–45, KF1–60, KF1–90 are increased to 144, 172 and 200 F/g (Fig. 4B), respectively, which is consistent with the variation tendency of specific surface area. KF4 or KF6 samples with over-abundant micropores are less effective in EDLC forming than KF1, which could be ascribed to the resistance of electrolyte ion transportation into the inner micropores.
In order to evaluate the cycle stability of the activated carbon materials, galvanostatic charge-discharge studies were performed at a fairly high current density of 1.0 A g−1 between −0.8 and 0 V in the 6 M KOH electrolyte. After 300 cycles, the capacitance retention of KF1–90 is as high as ∼98%, indicative of excellent electrochemical stability (Fig. 4D). Therefore the activated mesoporous carbon presents optimized micro-mesoporous structure for enhanced power density especially at high current density. In addition, abundant micropores and small mesopores in the original mesoporous walls generated by the activation treatment lead to large specific surface area (1410 cm3 g−1), which also provide low-resistant pathways, short distance for ions through the porous particles and made great contributions for EDLC forming.
4. Conclusion
Mesoporous carbon FDU-15 synthesized by a simple soft template technique has ordered mesostructure, low surface area of 660 m2 g−1 and uniform mesopores. Significantly, improvement in surface area up to 1410 m2 g−1 has been obtained through the KOH activation. Micropores generated after KOH activation provide large surface area and pore volume, small mesopores in the wall build various “bridges” interconnecting the main-mesopores and micropores. Such a micro-mesostructure facilitates ion transportation through mesopores, also increases efficiency of electrical double layer capacitor forming in micropores. The sample activated by KOH under the condition of KOH/carbon mass ratio of 1.0 and the activation time of 90 min, shows excellent electro-capacity performance of 200 F/g at the scan rate of 0.5 A/g. Such designed KOH activated ordered mesoporous carbon with ideal micro-mesopore structure can easily be obtained under optimized KOH activation condition, showing a great improvement in energy density and remain of power density, will be potentially used in commercial as mesoporous carbon based supercapacitors.Acknowledgements
This work was supported by the Fudan Startup Foundation for Advanced Talents, the NSF of China (20890123), the State Key Basic Research Program of the PRC (2009AA033701 and 2009CB930400), Science and Technology Commission of Shanghai Municipality (08DZ2270500), and Shanghai Leading Academic Discipline Project (B108). This work was also supported by Key Subjects Innovative Talents Program of Fudan University and Innovative. We greatly appreciate the financial supports from Delta Environmental & Educational Foundation (Taiwan).References
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS
.
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS
.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS
.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS
.
- A. Burke, J. Power Sources, 2000, 91, 37–50 CrossRef CAS
.
- E. Frackowiak and F. Beguin, Carbon, 2001, 39, 937–950 CrossRef CAS
.
- C. Largeot, C. Portet, J. Chmiola, P.-L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730–2731 CrossRef CAS
.
- G. Sun, J. Wang, X. Liu, D. Long, W. Qiao and L. Ling, J. Phys. Chem. C, 114, 18745–18751 CAS
.
- W. Xing, S. Z. Qiao, R. G. Ding, F. Li, G. Q. Lu, Z. F. Yan and H. M. Cheng, Carbon, 2006, 44, 216–224 CrossRef CAS
.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang, Z. Li, C. Yu, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS
.
- C. Xue, B. Tu and D. Zhao, Nano Res., 2009, 2, 242–253 CrossRef CAS
.
- A. Stein, Z. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265–293 CrossRef CAS
.
- J. Jin, S. Tanaka, Y. Egashira and N. Nishiyama, Carbon, 2010, 48, 1985–1989 CrossRef CAS
.
- K. Xia, Q. Gao, C. Wu, S. Song and M. Ruan, Carbon, 2007, 45, 1989–1996 CrossRef CAS
.
- A. B. Fuertes, G. Lota, T. A. Centeno and E. Frackowiak, Electrochim. Acta, 2005, 50, 2799–2805 CrossRef CAS
.
- X. Q. Wang, J. S. Lee, C. Tsouris, D. W. DePaoli and S. Dai, J. Mater. Chem., 2010, 20, 4602 RSC
.
- W. Xing, C. C. Huang, S. P. Zhuo, X. Yuan, G. Q. Wang, D. Hulicova-Jurcakova, Z. F. Yan and G. Q. Lu, Carbon, 2009, 47, 1715–1722 CrossRef CAS
.
- I. Villar, V. Ruíz, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Energy Fuels, 24, 3422–3428 Search PubMed
.
- T. Otowa, Y. Nojima and T. Miyazaki, Carbon, 1997, 35, 1315–1319 CrossRef CAS
.
- M. A. Lillo-Ródenas, J. Juan-Juan, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2004, 42, 1371–1375 CrossRef
.
- H. Shi, Electrochim. Acta, 1996, 41, 1633–1639 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c1jm12742j |
| This journal is © The Royal Society of Chemistry 2012 |
