Safety assessment of plant food supplements (PFS)†
Suzanne J. P. L.
van den Berg
*a,
Lluis
Serra-Majem
bc,
Patrick
Coppens
d and
Ivonne M. C. M.
Rietjens
a
aDivision of Toxicology, Wageningen University, Tuinlaan 5, 6703, HE Wageningen, The Netherlands. E-mail: Suzanne.vandenberg@wur.nl; Fax: +31 317 484931; Tel: +31 317 482756
bNutrition Research Foundation, University of Barcelona Science Park, 08028, Barcelona, Spain
cDepartment of Clinical Sciences, University of Las Palmas de Gran Canaria, 35080, Las Palmas, Spain
dEuropean Botanical Forum, Rue de l'Association 50, 1000 Brussels, Belgium
First published on 1st August 2011
Abstract
Botanicals and botanical preparations, including plant food supplements (PFS), are widely used in Western diets. The growing use of PFS is accompanied by an increasing concern because the safety of these PFS is not generally assessed before they enter the market. Regulatory bodies have become more aware of this and are increasing their efforts to ensure the safety of PFS. The present review describes an overview of the general framework for the safety assessment of PFS, focusing on the different approaches currently in use to assess the safety of botanicals and/or botanical compounds, including their history of safe use, the tiered approach proposed by the European Food Safety Authority (EFSA), the Threshold of Toxicological Concern (TTC) and the Margin of Exposure (MOE) concept. Moreover, some examples of botanical compounds in PFS that may be of concern are discussed. Altogether, it is clear that “natural” does not equal “safe” and that PFS may contain compounds of concern at levels far above those found in the regular diet. In addition, the traditional use of a PFS compound as a herb or tea does not guarantee its safety when used as a supplement. This points at a need for stricter regulation and control of botanical containing products, especially given their expanding market volume.
 Suzanne J. P. L. van den Berg | Suzanne J. P. L. van den Berg received her MSc degree in Biomedical Sciences from the Radboud University Nijmegen in September 2010 with a major in Toxicology, a minor in Nutrition and Health and a second minor in Human Pathobiology. After graduation, she started working as a PhD student at the department of Toxicology of Wageningen University (WUR), the Netherlands. Her current research is focused on the risk assessment of botanicals and botanical preparations used as plant food supplements. |
 Lluis Serra-Majem | Lluís Serra-Majem (Autonomous University of Barcelona) is an MD with a PhD in Nutrition, specialising in Preventive Medicine and Public Health. In 1995, he became Full Professor of Preventive Medicine & Public Health at the University of Las Palmas de Gran Canaria, and since 1997 he has chaired the Nutrition Research Foundation, University of Barcelona Science Park. He has been the Mediterranean Diet Foundation President since 1996, promoting the recognition of the Mediterranean diet as UNESCO Intangible Cultural Heritage of Humanity. He specialises in nutrition epidemiology and nutrient intake assessment. |
 Patrick Coppens | Patrick Coppens, graduate of the University of Louvain in Nutritional Sciences, is a leading expert in food law, with particular expertise in food safety, health claims and nutrition policy. He is a senior adviser in international food and health law, and scientific affairs at Brussels-based EAS, specialising in regulatory and strategic advice on nutritional products. In this capacity, he advises a number of trade bodies and is, in particular, Secretary General of the European Botanical Forum. He also presides over the task force on malnutrition of the Belgian Food and Health Plan. |
 Ivonne M. C. M. Rietjens | Professor Ivonne M. C. M. Rietjens is full professor in Toxicology at Wageningen University (WUR), Netherlands, member of the Royal Netherlands Academy of Arts and Sciences (KNAW), vice-chair of the ANS Panel of the European Food Safety Authority (EFSA), and member of several (international) advisory committees. She is author of over 260 scientific publications in refereed journals. Her research focuses on food ingredients, including natural toxins and functional food ingredients, physiologically-based kinetic and dynamic models for low dose cancer risk extrapolation, the consequences of genetic polymorphisms and life style factors for individual sensitivity and risk assessment, and alternative methods for animal testing. |
General introduction
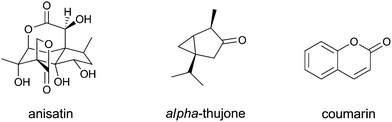
At present, there is an increasing interest for plant ingredients and their use in drugs, for teas, and/or in food supplements. Such use may result in intake levels that exceed the normal dietary intake of these botanicals and/or their ingredients. Although plant food supplements (PFS) are widely marketed, the safety of these PFS is not generally assessed before they enter the market. Regulatory bodies have become more aware of this and are increasing their efforts to ensure the safety of botanical supplements.1,2 The Scientific Committee of the European Food Safety Authority (EFSA) issued a guidance in which a general framework for the safety assessment of botanicals and botanical preparations was presented.3 In this opinion, it was recommended to test the proposed approach for the safety assessment of botanicals and botanical preparations with a number of examples, resulting in advice on the EFSA guidance document, based on real case studies by the EFSA Scientific Cooperation (ESCO) working group on botanicals and botanical preparations,4,5 and an updated guidance document.6In spite of this regulatory awareness, it cannot be excluded that specific developments may still result in health concerns. Such developments include; (i) overconsumption by particular groups, sometimes stimulated by companies making misleading claims on their websites or in their literature; (ii) the fact that many consumers equate “natural” with “safe” when considering PFS; (iii) the availability of potentially harmful PFS through internet sites from countries where regulations are not in place; and (iv) the fact that often there are no detailed requirements on safety and quality of PFS. The latter is especially worrying as botanicals are known to be of variable quality with high variation, not only in the content of the active ingredients but also of the toxic principles, and the fact that several cases of the replacement of a harmless variety with a toxic alternative have already occurred. One such example relates to Chinese star anise (Illicium verum) used in many cultures, mostly for preparing tea. In September 2001 in The Netherlands, more than 60 people showed nausea and vomiting after drinking a herbal tea called ‘starmix tea’ containing star anise, and 22 people were hospitalized due to tonic-clonic insults.7,8 Electroencephalograms (EEGs) showed epileptiform abnormalities, indicating a diffuse cerebral disease.9 The complaints were ascribed to a toxic star anise species comparable to Japanese star anise (Illicium anisatum) containing anisatin, which was accidentally exchanged for the non-toxic Chinese star anise (Illicium verum).7 After this incident and the detection of Japanese star anise in consignments of star anise from third countries, the EU took legislative measures to increase control and safeguard public health (Commission Decision 2002/75EC). These measures included the requirement for documentary evidence confirming that the imported products do not contain any Japanese star anise, and random sampling and analysis of these products. Sampling and control was also implemented for products already on the market. These measures were of a temporal nature as they were lifted one year later given the absence of new cases of contamination and poisoning (Commission Decision 2003/602/EC).
Another example of the replacement of a harmless variety with a toxic alternative comes from the use of Chinese medical products. In 1991, a unique form of nephropathy was reported in Belgium. Over 100 young women suffered from kidney damage, developing into cancer of the kidneys and the urinary tract in several patients.10,11 This adverse effect was associated with the prolonged intake of a Chinese herb-based weight loss preparation in which Stephania tetranda was accidentally replaced by Aristolochia fanchi, because both plants are used under the same name ‘Fangji’ in Chinese folk medicine.11 On the basis of this incident, risk management actions have been taken by the authorities of most EU Member States to prevent such an incident from happening again.
The present review focuses on the general framework for safety assessment of PFS, also presenting some examples of types of botanical compounds of concern and their major adverse effects. Furthermore, some emphasis is given to the modulating effect of other PFS ingredients modifying the actual risk posed by a specific botanical ingredient.
General framework for risk/safety assessment of PFS
At present, a formalized framework for the safety assessment of PFS is not in place and safety assessments are performed on a national basis or by dedicated bodies like, for example, the EMA (former EMEA) (European Medicines Agency). The EMA, however, judges the safety of medicinal preparations and does not refer to the safety of PFS in the field of food use.In 2009, the Scientific Committee of the EFSA published an updated guidance on the scientific data needed to carry out a safety assessment of a botanical or a botanical preparation.6 This guidance proposes a two-tiered scientific approach for the safety assessment depending on the available knowledge on a given botanical and the substance(s) it contains. The tiered approach takes into account the nature of the botanical or botanical preparation, its intended uses and levels of use including PFS and whether the botanical or botanical preparation has a long term (traditional) history of food use, showing that, at proposed exposure levels, no adverse effect on human health has been reported (Tier 1). In addition it is indicated that for botanical compounds lacking a history of food use, or for botanicals whose intended use levels will significantly exceed historical intake levels, an assessment of safety based on experimental toxicity data may be required (Tier 2). This further safety assessment may focus on a specific compound if the compound of concern in a PFS can be well defined. In this case, its safety may be judged based on existing safety values for that ingredient, such as the Acceptable Daily Intake (ADI) or Tolerable Daily Intake (TDI). An example would be evaluating the intake of trans-anethole from the use of bitter fennel fruits using the temporary ADI of 0–2.0 mg kg−1 bw for trans-anethole.4,12
The EFSA document does not give clear guidance on what to do when no health-based guidance values are available, but states that consideration of exposure to the substance of concern in relation to the Threshold of Toxicological Concern (TTC) values may be helpful. Thus, one may suggest that in cases where no health-based guidance values are available, the TTC approach could be used. The TTC concept is an approach that aims to establish a human exposure threshold value below which there is a very low probability of an appreciable risk to human health. This TTC approach compares the estimated oral intake with a TTC value derived from chronic oral toxicity data for structurally-related compounds. The TTC values for the so-called Cramer structural classes13 were established by Munro et al.14,15 based on an analysis of data from chronic toxicity studies on 137, 28 and 448 compounds in the Cramer classes I, II and III, respectively. Based on the 5th percentile values for the NOAEL distributions for each class of compounds and the application of a 100-fold uncertainty factor, the corresponding human exposure threshold values were calculated. These analyses gave thresholds of toxicological concern of 1800, 540 and 90 μg per person per day for structural classes I, II and III, respectively, equivalent to 30, 9 and 1.5 μg kg−1 bw per day for a 60 kg person.16 When the estimated intake of a PFS ingredient of concern is below the TTC of its respective class, this can be used to conclude on the safety of its proposed use and use levels.
In cases where the botanical ingredient contains substances that are both genotoxic and carcinogenic, assessment of the risk to human health is complicated, and an international scientific agreement concerning the best strategy for the risk assessment of genotoxic and carcinogenic compounds is lacking.17 As a result, a variety of approaches are used by different regulatory and advisory bodies. While some offer qualitative advice, others present quantitative approaches with respect to the risk assessment of genotoxic carcinogens.18 The advice that the intake of a particular substance should be as low as reasonably achievable (ALARA) is a qualitative approach that is widely used.19,20 Nevertheless, the use of this qualitative approach can be considered to be of limited value since it does not define the priorities necessary for risk management actions.17,21 Furthermore, this approach does not include data on carcinogenic potency nor data on human exposure.20,21 As a consequence, this approach might be applied to compounds even though their current exposure levels are of no risk to human health.
Quantitative approaches often include dose-response data, derived from epidemiological studies or rodent carcinogenicity bioassays and exposure data to estimate the risk to human health.20 However, risk estimates for a particular compound may show variable outcomes depending more on the selected mathematical model than on the experimental data,17,20 and the use of numerical risk estimates may be interpreted incorrectly.20 Considering the possible uncertainties and existing disadvantages connected to the use of qualitative and quantitative approaches such as ALARA and low-dose cancer risk extrapolation, the use of a Margin of Exposure (MOE) approach was recommended by expert groups of the EFSA, the Joint FAO/WHO expert committee on Food Additives (JECFA) and the International Life Sciences Institute (ILSI).17,19–21 The MOE is a dimensionless ratio based on a reference point obtained from epidemiologic or experimental data on tumor incidence, which is divided by the estimated daily intake in humans.17 Thus, the MOE approach compares toxic effect levels with human exposure levels. The MOE approach is considered a useful and pragmatic option for risk assessment of substances that may be both genotoxic and carcinogenic.17,19 It allows comparison between compounds and prioritization of risk management actions, especially if the calculation of the MOE is accompanied by an appropriate narrative explaining inherent uncertainties.
Alternatively, one may evaluate whether the expected exposure to the genotoxic and carcinogenic ingredient is likely to be increased, compared to the intake from other sources. We propose that another option would be to apply the TTC defined for genotoxic compounds, of 0.15 μg per person per day corresponding to 0.0025 μg kg−1 bw per day for a 60 kg person.22 This level reflects a low probability of a lifetime cancer risk, greater than one in a million based on linear extrapolation of the TD50 values from rodent carcinogenicity studies on 730 structurally-related compounds. Noteworthily for the so-called high potency genotoxic chemicals (i.e. aflatoxin-like compounds, N-nitroso compounds and azoxy compounds), it is suggested that a TTC should not be considered since compound-specific risk assessment is required for this group.22 At present, there is an EFSA working group on Thresholds of Toxicological Concern (http://www.efsa.europa.eu/en/sc/scwgs.htm) and this working group may give further guidance on the use of the TTC approach.
Prioritization of botanicals of possible concern
The guidance document published by the Scientific Committee of the EFSA6 provides a set of criteria to help prioritize the safety assessment of botanical compounds that are in use. The document states that; “Priority should be given to botanicals and botanical preparations: i) known to have an established history of food use and that have been identified to contain significant levels of substances of concern, ii) that are not allowed/recommended for food use in some European countries, but which are still in use in some other EU countries, particularly when the intended use levels in food are known or expected to be high, iii) for which some adverse health effects have been reported, either anecdotally, or on the basis of case reports of intoxication, epidemiological data or any toxicity data from livestock animals or experimental animals, or for botanicals that closely resemble botanicals which are known to have caused toxic effects, iv) for which consumption has significantly increased during recent years in Member States, v) for which there are both limited history of use and toxicity data available, and for which the intended use levels are expected to be relatively high (e.g. high interest to the food industry). Botanical ingredients that are reported to have a low toxic potential, and for which the intended intake/exposure levels are within the range of intake levels resulting from the European Member States average diet would be given a low priority.”The EFSA has also compiled the available information on a large number of botanicals that have been reported to contain substances that may be of health concern when specific parts are used and/or inadequate processing procedures are used in making botanical extracts and/or botanical products. The resulting compendium can be found on the internet23 and will be regularly updated.
Examples of PFS compounds of possible concern because of their genotoxic and carcinogenic properties
Botanicals and/or botanical preparations may contain compounds of concern because of their genotoxic and carcinogenic properties like, for example, compounds belonging to the groups of pyrrolizidine alkaloids, alkenylbenzenes or aristolochic acids.Pyrrolizidine alkaloids (Fig. 1) are converted by cytochromes P450 to pyrrolic dehydro-alkaloid metabolites that alkylate DNA and other macromolecules, causing liver cell necrosis and liver cancer.24,25 The use of toxic pyrrolizidine alkaloid-containing botanicals as food or food products is restricted in several countries around the world. Nevertheless, there is no consensus in these regulations. South Africa, the UK, Belgium, Australia and New Zealand restrict the internal use of comfrey and products derived from this botanical, and also completely prohibit the use of several other toxic pyrrolizidine alkaloid-containing plants in food, such as Senecio spp., Symhytym spp., Crotalaria spp. and Heliotropium spp.26–29 The use of pyrrolizidine alkaloids in PFS is still allowed in The Netherlands, as well as in Germany, although limitations have been adopted in these countries with regard to the exposure to toxic pyrrolizidine alkaloids resulting from the use of herbal supplements.30,31 Following the Dutch ‘Warenwetbesluit Kruidenpreparaten’, as adopted in January 2001, the total content of pyrrolizidine alkaloids present in botanical supplements may not exceed 1 μg kg−1.30 In Germany, the exposure to pyrrolizidine alkaloids may not exceed 0.1 μg day−1 when pyrrolizidine alkaloids containing botanical products for oral use are consumed for a period longer than 6 weeks.31 When the exposure period does not exceed this limit of 6 weeks, an exposure of 1 μg pyrrolizidine alkaloids per day is allowed resulting from the use of herbal medicines.31 Furthermore, the U.S. Food and Drug Administration (FDA) raised serious concerns for human health with regard to the use of pyrrolizidine alkaloids, and ‘the agency strongly recommends that firms marketing a product containing comfrey or another source of pyrrolizidine alkaloids remove the product from the market and alert its customers to immediately stop using the product’.32 Within the same line of reasoning, Health Canada advised the Canadian population not to use any comfrey containing products.33
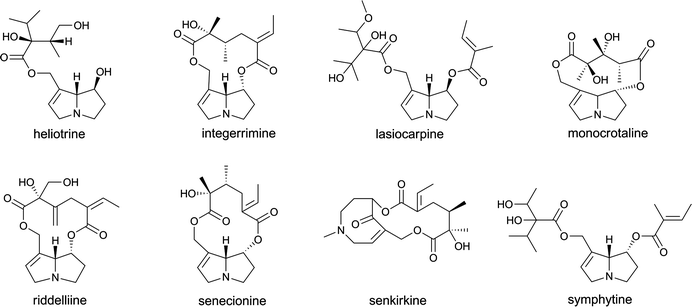 | ||
| Fig. 1 The structural formulae of several pyrrolizidine alkaloids. | ||
Alkenylbenzenes including apiole, β-asarone, elemicin, estragole, methyleugenol, myristicin and safrole (Fig. 2) are converted by cytochromes P450 and sulfotransferase (SULT)-mediated biotransformation to genotoxic and carcinogenic 1′-sulfooxymetabolites that bind to DNA and cause liver cancer.34–38 Although the use of estragole, methyleugenol (Regulation (EC) no. 1334/2008 of the European Parliament and of the Council, 16 December 2008), safrole and β-asarone (Council Directive 88/388/EEC of 22 June 1988) as pure compounds in food is prohibited within the EU because of their genotoxic and carcinogenic potentials, currently no harmonized restrictions have been made in the EU with regard to the use of alkenylbenzene-containing botanicals in PFS. In countries where no restrictions are applicable, PFS containing high levels of alkenylbenzenes may be on the market.
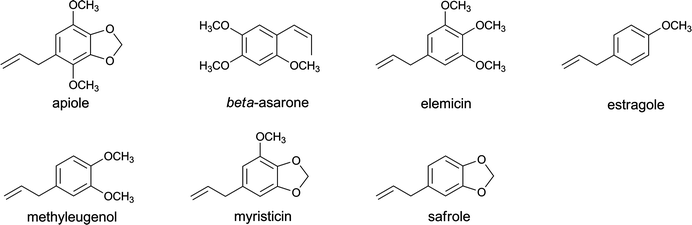 | ||
| Fig. 2 The structural formulae of important alkenylbenzenes. | ||
It is also important to stress that despite prohibitions, products containing compounds of concern may still be offered on the market. An example of the occurrence of prohibited compounds in botanical preparations is the presence of aristolochic acids in Chinese herbal preparations used in traditional Chinese medicine. It was previously demonstrated that several Chinese herbal preparations that are sold on the Dutch market still contain aristolochic acids that are prohibited worldwide.39 Aristolochic acid I and II (Fig. 3) are converted by reductive metabolic activation by cytochromes P450 and/or by other enzymes, resulting in formation of reactive nitrenium ion metabolites that cause Chinese Herb Nephropathy and urothelial cancers.24,40 In 190 Chinese traditional herbal preparations sampled between 2002 and 2006 on the Dutch market, aristolochic acid I was found in 25 samples up to a concentration of 1676 mg kg−1, and aristolochic acid II was found in 13 of these samples up to 444 mg kg−1.39
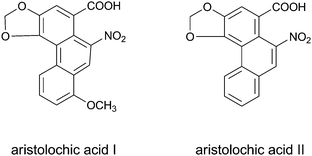 | ||
| Fig. 3 The structural formulae of aristolichic acid I and II. | ||
Examples of PFS compounds of possible concern because of neurotoxicity
Another group of PFS compounds may be of concern because of their neurotoxicity. These PFS compounds include, for example, ephedrine analogues anisatin and α-thujone. These compounds appear to interact with one of the neurotransmitter systems.Ephedrine and ephedrine analogues including pseudo-ephedrine, norephedrine, methylephedrine and norpseudo-ephedrine may act as adrenalin agonists. Fig. 4 presents the structural formulae of these compounds. PFS containing botanicals like Ephedra sinica, Ephedra intermedia and Ephedra equisatine, also known by their Chinese name “Ma Huang”, contain these compounds and are used for the improvement of weight loss and athletic performance.41 As adrenalin agonists, these compounds produce a sympathomimetic response, characterized by increased heart rhythm, hypertension (elevated blood pressure) and central nervous system stimulation. p-Synephrine (Fig. 4) also acts as an adrenalin agonist. p-Synephrine is the main active principle found in the fruit of several citrus species, including Citrus aurantum and Citrus reticulata. In traditional Chinese medicine, the fruit is also known as Chih-shih. Evaluations concerning preparations containing high amounts of p-synephrine concluded that there may be a possible safety concern,42,43 and extracts used in many dietary supplements and herbal weight-loss formulas as an alternative to Ephedra have concentrations of p-synephrine that are much higher than the p-synephrine concentrations reported for traditional extracts of the dried fruit or peel.5 This reflects another important issue to be taken into account when assessing the safety of PFS, i.e. that some preparations of a botanical may be marketed containing significantly higher levels of active (toxic) principles than those normally occurring in historical food uses of the same botanical. Furthermore, the position isomer of synephrine found in bitter orange (Citrus aurantium L. ssp. aurantium L.) peel is p-synephrine, not m-synephrine. The presence of any amount of m-synephrine, higher amounts of the (+)-p-synephrine stereoisomer or higher amounts of octopamine in PFS, supposedly containing only extracts of bitter orange, should be considered undesirable and suspicious of adulteration, thus strongly suggesting a requirement for a more efficient quality control.5
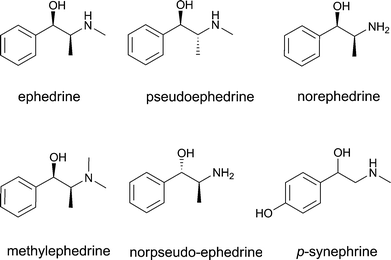 | ||
| Fig. 4 The structural formula of ephedrine and its analogues pseudoephedrine, norephedrine, methylephedrine, norpseudo-ephedrine and p-synephrine. | ||
Anisatin (Fig. 5), the toxic ingredient in Japanese star anise (Illicium anisatum) that was accidentally exchanged for the non-toxic Chinese star anise (Illicium verum),7 acts as a non-competitive γ-amino butyric acid (GABA) antagonist that can cause tonic-clonic insults.44
The terpenoid α-thujone (Fig. 5) occurs in the essential oils and parts of the plants Artemesia absinthum (wormwood), Salvia officinalis (sage), Salvia scarea (clary), Tanacetum vulgaris (tansy), and in Juniperus and Cedris spp., which may occur in PFS. The mechanism of neurotoxicity of α-thujone has been ascribed to the fact that it blocks the receptors for γ-aminobutyric acid (GABA).45
Examples of PFS compounds of possible concern because of other modes of action
PFS compounds of concern because of other modes of action include, for example, kavalactones, cyanogenic glycosides and coumarin.Kavalactones originate from the rootstock of the kava (Piper methysticum) plant. The structural formulas of kavain, dihydrokavain, methysticin, dihydromethysticin, yangonin and desmethoxy-yangonin, which are the main kavalactones found in Piper methysticum, are shown in Fig. 6. Through their action on the nervous system, kavalactones exert sedative, analgesic, anticonvulsant and muscle relaxant effects. The mechanism of action for this effect may include inhibition of monoamine oxidase (MAO) activity, inhibition of noradrenalin re-uptake in the presynaptic neuron and/or action as a dopamine antagonist.46,47 The major toxic side effects of kava kava are dermopathy and liver toxicity. The liver toxicity may be related to glutathione depletion and/or quinone formation.24,48,49 Since 1999, cases of severe hepatic toxicity in people using kava-containing herbal products have been reported in Europe and the United States,50,51 including several cases in which patients required a liver transplant following the use of kava containing products.51 As a result, the use of kava kava is now prohibited in PFS and medicinal products in most countries.
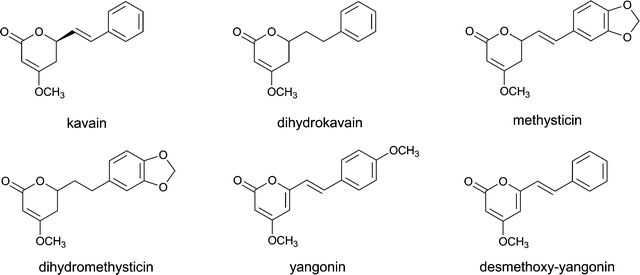 | ||
| Fig. 6 The structural formulae of the main kavalactones found in Piper methysticum. | ||
Cyanogenic glycosides are present in a number of food plants and seeds, and include compounds like amygdalin, dhurrin, linamarin, linustatin, lotaustralin, neolinustatin, prunasin and taxiphyllin (Fig. 7). Products containing high levels of amygdalin are sold via the internet as vitamin B17 or laetrile for cancer treatment, although many countries, including the USA, UK, Singapore and The Netherlands have banned its sale.52–54 Cyanogenic glycosides are a cause of concern because once ingested they are metabolized to cyanide. Cyanide is released from the cyanogenic glycosides by plant β-glucosidases that come into contact with the cyanogenic glycosides when the fresh plant material is macerated as in chewing, or by β-glucosidases present in the gut flora. Cyanide causes toxic effects by binding to cytochrome oxidase, the terminal enzyme in the mitochondrial electron transport chain. By hampering the generation of ATP and oxygen utilization, a histotoxic anoxia is produced.
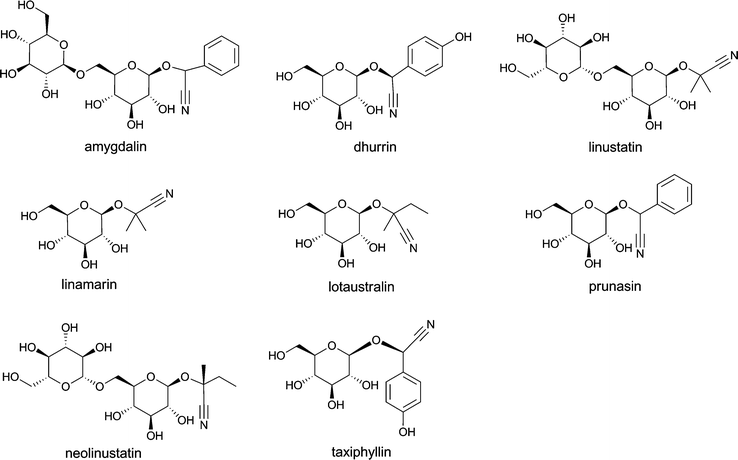 | ||
| Fig. 7 The structural formulae of important cyanogenic glycosides. | ||
Cinnamon-containing PFS may contain high levels of coumarin (Fig. 5), which causes liver damage and, at high dose levels, liver tumours by a non-genotoxic mechanism. Coumarin induces tumours via a mechanism of action that is preceded by toxicity in the target organ and consequently a TDI of 0.1 mg kg−1 bw per day has been defined.55 This TDI can be used to judge the safety of not only coumarin-containing foods but also of coumarin-containing PFS.
Examples of PFS compounds of possible concern because of their interactions with prescription drugs
PFS may contain compounds interacting with the pharmacokinetics and/or pharmacodynamics of prescription drugs when concomitantly consumed, presenting a potential safety issue. The most common botanical–drug interactions that have been described involve botanicals like ginkgo biloba (Ginkgo biloba L), kava kava (Piper methysticum), black cohosh (Acteae racemosa), gingseng (Panax gingseng) and St. John's wort (Hypericum perforatum), with the latter example being studied the most thoroughly.St. John's wort is used to treat a variety of diseases such as anxiety, mild to moderate depression, sleeping disorders and obsessive-compulsive disorder. The antidepressant effects of St. John's wort are thought to be caused by its active ingredient, hyperforin, inhibiting the synaptosomal uptake of serotonin, norepinephrine and dopamine.56 Several interactions with prescribed drugs have been reported for St. John's wort and a number of studies indicate that changes in the activity of specific cytochromes P450 may underlie these botanical–drug interactions.57–60 As a result of the potent inhibition of these metabolising enzymes, the plasma levels of prescribed drugs including alprazolam, irinotecan and indinavir might be decreased after their concurrent use with St. John's wort.58–60 In 2000, the FDA published a health advisory instructing health care professionals to report potentially adverse effects related to the use of St. John's wort in combination with prescription drugs.61
Most of the current evidence for botanical–drug interactions is based on case reports while experimental data are limited. To date, possible botanical–drug interactions are often not indicated on the label of PFS nor the drug, making it difficult for consumers to make an informed decision. Moreover, interactions are not frequently ascribed to the effects of natural products, as these products are often regarded as ‘safe’. Therefore, the management of botanical–drug interactions should take an important position in promoting the safe use of PFS, underlining awareness of possible interactions between PFS and prescribed drugs. Currently, a database is being prepared providing an overview of the existing data, but also producing new data, including the interactions between botanicals and prescribed drugs (http://www.plantlibra.eu/web) to assist in monitoring the safety of PFS, and facilitating information to consumers and health care providers to create more awareness of possible botanical–drug interactions.
Matrix effects modulating toxicity
When evaluating the safety of PFS compounds, it is important to consider that the kinetics and/or the toxicity of a PFS ingredient could be modified by the matrix in which it is present. This could result in the toxicity being unchanged, reduced or even increased. An example of increased toxicity can be found in the fact that epigallocatechin gallate (EGCG) given in a green tea extract to rats appears to be eliminated less readily from the body and to have a higher toxicity than when given as a pure compound.62,63 An example of decreased toxicity is presented by the reduction of the level of DNA binding of the proximate carcinogenic metabolite 1′-hydroxyestragole by a methanolic basil extract.64,65 This inhibition by the basil extract was shown to occur at the level of the SULT-mediated bioactivation of 1′-hydroxyestragole to 1-sulfoxyestragole and to be mediated by the flavonoid nevadensin present in basil at a high level.64,65Whenever a matrix effect is advocated to support the safety of specific levels of PFS compounds (e.g. that data from a pure compound may overestimate effects of the compound in the botanical matrix), data should be provided to demonstrate the occurrence of the matrix effect of the preparation and its magnitude. It is also important to realise that when a matrix effect is demonstrated for an interacting botanical, the matrix effect for the PFS may be different. Thus, the matrix effect should be taken into account in the safety assessment of PFS compounds on a case-by-case basis.
Conclusions
PFS on the market may contain active compounds that are of concern. For several compounds, regulatory authorities are aware of the problems encountered and have taken or are considering appropriate regulatory actions to protect the public. These regulatory actions may vary from setting TDIs (such as, for example, for coumarin and trans-anethole), setting restrictions on use (such as for estragole, methyleugenol, safrole and β-asarone), informing the public to be cautious and aware of possible adverse side effects (as for kava kava-derived kavalactones) or taking specific plant varieties and/or their compounds off the market (such as plants containing aristolochic acids, toxic pyrrolizidine alkaloids and kavalactones).However, at present, a general framework for the safety assessment of PFS is not in place and safety assessments are performed on a national and ad hoc basis, often following incidents. Besides, even when regulatory measures are in place, PFS-containing compounds of concern may still be offered for sale on the internet, such as in case of the cyanogenic glycoside amygdalin (vitamin B17) or PFS containing high levels of alkenylbenzenes. Altogether, it is clear that “natural” does not equal “safe” and that PFS may contain compounds of concern at levels far above those found in the regular diet. In addition, the traditional use of a PFS compound as an herb or tea does not guarantee its safety when used as a supplement. This points to a need for stricter regulation and control of botanical-containing products, especially given their expanding market volume.
Acknowledgements
The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 245199. It has been carried out within the PlantLIBRA project (web site: http://www.plantlibra.eu). This report does not necessarily reflect the Commission’s views or its future policy in this area.Notes and references
- Discussion paper on botanicals and botanical preparations widely used as food supplements and related products: coherent and comprehensive risk assessment and consumer information approaches, EFSA, Brussels, 2004. URL: http://www.efsa.europa.eu/fr/efsajournal/doc/1249.pdf Search PubMed.
- D. A. Taylor, Environ. Health Perspect., 2004, 112, A750–A753 Search PubMed.
- Draft guidance document of the Scientific Committee for the safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, EFSA, 2007, URL: http://www.oeti.hu/download/sc_guidance_bota_public_consultation.pdf Search PubMed.
- Advice on the EFSA guidance document for the safety assessment of botanicals and botanical preparations intended for use as food supplements, based on real case studies, EFSA, Parma, 2009. URL: http://www.efsa.europa.eu/en/supporting/doc/280r.pdf Search PubMed.
- G. Speijers, B. Bottex, B. Dusemund, A. Lugasi, J. Toth, J. Amberg-Muller, C. L. Galli, V. Silano and I. M. C. M. Rietjens, Mol. Nutr. Food Res., 2010, 54, 175–185 Search PubMed.
- Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, EFSA, 2009. URL: http://www.efsa.europa.eu/fr/efsajournal/doc/1249.pdf Search PubMed.
- E. S. D. Johanns, L. E. van der Kolk, H. M. A. van Gemert, A. E. Sijben, P. W. Peters and I. de Vries, Ned. Tijdschr. Geneeskd., 2002, 146, 813–816 Search PubMed.
- A. M. Oudesluys-Murphy and N. Oudesluys, Lancet, 2002, 360, 878 Search PubMed.
- G. J. Biessels, F. H. Vermeij and F. S. S. Leijten, Ned. Tijdschr. Geneeskd., 2002, 146, 808–811 Search PubMed.
- M. Vanhaelen, R. Vanhaelen-Fastre, P. But and J. L. Vanherweghem, Lancet, 1994, 343, 174 Search PubMed.
- J. L. Vanherweghem, M. Depierreux, C. Tielemans, D. Abramowicz, M. Dratwa, M. Jadoul, C. Richard, D. Vandervelde, D. Verbeelen and R. Vanhaelen-Fastre et al., Lancet, 1993, 341, 387–391 Search PubMed.
- Safety evaluation of certain food additives, prepared by the 51st meeting of JECFA, FAS 42-JECFA 51/5, JECFA, 1998 Search PubMed.
- G. M. Cramer, R. A. Ford and R. L. Hall, Food Cosmet. Toxicol., 1976, 16, 255–276 Search PubMed.
- I. C. Munro, R. A. Ford, E. Kennepohl and J. G. Sprenger, Food Chem. Toxicol., 1996, 34, 829–867 CrossRef CAS.
- I. C. Munro, E. Kennepohl and R. Kroes, Food Chem. Toxicol., 1999, 37, 207–232 Search PubMed.
- R. Kroes, A. G. Renwick, V. Feron, C. L. Galli, M. Gibney, H. Greim, R. H. Guy, J. C. Lhuguenot and J. J. M. van de Sandt, Food Chem. Toxicol., 2007, 45, 2533–2562 Search PubMed.
- Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic, EFSA, 2005. URL: http://www.efsa.europa.eu/en/efsajournal/doc/282.pdf Search PubMed.
- S. Barlow and J. Schlatter, Toxicol. Appl. Pharmacol., 2010, 243, 180–190 Search PubMed.
- S. Barlow, A. G. Renwick, J. Kleiner, J. W. Bridges, L. Busk, E. Dybing, L. Edler, G. Eisenbrand, J. Fink-Gremmels, A. Knaap, R. Kroes, D. Liem, D. J. G. Muller, S. Page, V. Rolland, J. Schlatter, A. Tritscher, W. Tueting and G. Wurtzen, Food Chem. Toxicol., 2006, 44, 1636–1650 Search PubMed.
- J. O'Brien, A. G. Renwick, A. Constable, E. Dybing, D. J. G. Muller, J. Schlatter, W. Slob, W. Tueting, J. van Benthem, G. M. Williams and A. Wolfreys, Food Chem. Toxicol., 2006, 44, 1613–1635 Search PubMed.
- Summary and conclusions of the sixty-fourth meeting of JECFA, JECFA, Rome, 2005, URL: ftp://ftp.fao.org/es/esn/jecfa/jecfa64_summary.pdf Search PubMed.
- R. Kroes, A. G. Renwick, M. Cheeseman, J. Kleiner, I. Mangelsdorf, A. Piersma, B. Schilter, J. Schlatter, F. van Schothorst, J. G. Vos and G. Wurtzen, Food Chem. Toxicol., 2004, 42, 65–83 CrossRef CAS.
- Compendium of botanicals that have been reported to contain toxic, addictive, psychotropic or other substances of concern on request of EFSA, EFSA, Parma, 2009. URL: http://www.efsa.europa.eu/en/supporting/doc/280rax1.pdf Search PubMed.
- I. M. C. M. Rietjens, M. J. Martena, M. G. Boersma, W. Spiegelenberg and G. M. Alink, Mol. Nutr. Food Res., 2005, 49, 131–158 Search PubMed.
- IPCS environmental health criteria 80, Pyrrolizidine alkaloids, WHO, 1988, URL: http://www.inchem.org/documents/ehc/ehc/ehc080.htm Search PubMed.
- Koninklijk besluit van 29 augustus 1997 betreffende de fabricage van en de handel in voedingsmiddelen die uit planten of plantenbereidingen samengesteld zijn of deze bevatten, Belgian State Journal, 21 November 1997, URL: http://www.bbkbio.be/pdf/KB290897_geconsolideerde-versie-op-28022005_NL.pdf Search PubMed.
- The Medicines (Products Other Than Veterinary Drugs) (General Sale List), Amendment Order 1994 no. 2410, United Kingdom, 1994. URL: http://www.legislation.gov.uk/uksi/1994/2410/made Search PubMed.
- Regulations relating to the prohibition of comfrey and comfrey containing foodstuffs and jelly confectionery containing konjac in foodstuffs - Foodstuffs, Cosmetics and Disinfectants Act (54/1972), South African Department of Health, 2002, URL: http://www.doh.gov.za/docs/regulations/2002/reg1541.html Search PubMed.
- Standard 1.4.4 prohibited and restricted plants and fungi, Australia New Zealand Food Standards, 2009, URL: http://www.comlaw.gov.au/Details/F2009C00806 Search PubMed.
- Besluit van 19 januari 2001, Houdende vaststelling van het Warenwetbesluit Kruidenpreparaten, Staatsblad van het Koninkrijk der Nederlanden, 2001, 56, 1–12 Search PubMed.
- Bundesrepublik Deutschland/pyrrolizidine alkaloide, stufe ii abwehr von arzneimittelrisiken, Pharm. Ztg. 1992, 137, 2088–2089 Search PubMed.
- FDA Advises Dietary Supplement Manufacturers to Remove Comfrey Products From the Market, FDA, 2001, URL: http://www.fda.gov/Food/DietarySupplements/Alerts/ucm111219.htm Search PubMed.
- Health Canada advises consumers not to use or ingest the herb comfrey or health products that contain comfrey, Health Canada, 2003, http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/public/_2003/index-eng.php Search PubMed.
- S. M. F. Jeurissen, A. Punt, M. G. Boersma, J. J. Bogaards, Y. C. Fiamegos, B. Schilter, P. J. van Bladeren, N. H. Cnubben and I. M. C. M. Rietjens, Chem. Res. Toxicol., 2007, 20, 798–806 Search PubMed.
- D. H. Phillips, J. A. Miller, E. C. Miller and B. Adams, Cancer Res., 1981, 41, 176–186 Search PubMed.
- R. L. Smith, T. B. Adams, J. Doull, V. J. Feron, J. I. Goodman, L. J. Marnett, P. S. Portoghese, W. J. Waddell, B. M. Wagner, A. E. Rogers, J. Caldwell and I. G. Sipes, Food Chem. Toxicol., 2002, 40, 851–870 Search PubMed.
- R. W. Wiseman, T. R. Fennell, J. A. Miller and E. C. Miller, Cancer Res., 1985, 45, 3096–3105 Search PubMed.
- R. W. Wiseman, E. C. Miller, J. A. Miller and A. Liem, Cancer Res., 1987, 47, 2275–2283 Search PubMed.
- M. J. Martena, J. C. A. van der Wielen, L. F. J. van de Laak, E. J. M. Konings, H. N. de Groot and I. M. C. M. Rietjens, Anal. Bioanal. Chem., 2007, 389, 263–275 CrossRef CAS.
- G. Gillerot, M. Jadoul, V. M. Arlt, C. van Ypersele De Strihou, H. H. Schmeiser, P. P. But, C. A. Bieler and J. P. Cosyns, Am. J. Kidney Dis., 2001, 38, E26 Search PubMed.
- P. Shekelle, M. L. Hardy, S. C. Morton, M. Maglione, M. Suttorp, E. Roth, L. Jungvig, W. A. Mojica, J. Gagne, S. Rhodes and E. McKinnon, Evid. Rep. Technol. Assess. (Summ.), 2003, 1–4 Search PubMed.
- G. Calapai, F. Firenzuoli, A. Saitta, F. Squadrito, M. R. Arlotta, G. Costantino and G. Inferrera, Fitoterapia, 1999, 70, 586–592 CrossRef CAS.
- S. Jack, T. Desjarlais-Renaud and K. Pilon, Can. Adverse React. Newslett., 2007, 17, 2–3 Search PubMed.
- E. Kakemoto, E. Okuyama, K. Nagata and Y. Ozoe, Biochem. Pharmacol., 1999, 58, 617–621 Search PubMed.
- K. M. Hold, N. S. Sirisoma, T. Ikeda, T. Narahashi and J. E. Casida, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3826–3831 CrossRef CAS.
- L. Schelosky, C. Raffauf, K. Jendroska and W. Poewe, J. Neurol., Neurosurg. Psychiatry, 1995, 58, 639–640 Search PubMed.
- J. J. Spollen, S. M. Spollen and J. S. Markowitz, J. Pharm. Pract., 1999, 12, 196–209 Search PubMed.
- B. M. Johnson, S. X. Qiu, S. Zhang, F. Zhang, J. E. Burdette, L. Yu, J. L. Bolton and R. B. van Breemen, Chem. Res. Toxicol., 2003, 16, 733–740 Search PubMed.
- P. A. Whitton, A. Lau, A. Salisbury, J. Whitehouse and C. S. Evans, Phytochemistry, 2003, 64, 673–679 Search PubMed.
- M. Escher, J. Desmeules, E. Giostra and G. Mentha, Br. Med. J., 2001, 322, 139 Search PubMed.
- Centers for disease and cancer prevention, MMWR Morb. Mortal Week. Rep., 2002, 51, 1065–1067 Search PubMed.
- Import Alert 62–01, FDA, 2009, URL: http://www.accessdata.fda.gov/cms_ia/importalert_167.html Search PubMed.
- Statutory Instrument (SI) 1984:87, The Medicines (Cyanogenetic Substances) Order 1984 no. 187, United Kingdom, 1984, URL: http://www.legislation.gov.uk/uksi/1984/187/made Search PubMed.
- Sale of Drugs (Prohibited Drugs) (Consolidation) Regulations, Health Sciences Authority, Singapore, 1985, URL: http://www.hsa.gov.sg/publish/etc/medialib/hsa_library/health_products_regulation/legislation/sale_of_drugs_act.Par.6933.File.dat/SALE%20OF%20DRUGS%20(PROHIBITED%20DRUGS)%20(CONSOLIDATION)%20REGULATIONS.pdf Search PubMed.
- Opinion of the Scientific Panel on Food additives, Flavourings, processing aids and materials in contact with food (AFC), on a request from the commission related to Coumarin, EFSA, The EFSA Journal, 2004, 104, 1–36. URL: http://www.efsa.europa.eu/en/scdocs/doc/104.pdf Search PubMed.
- S. S. Chatterjee, S. K. Bhattacharya, M. Wonnemann, A. Singer and W. E. Muller, Life Sci., 1998, 63, 499–510 CrossRef CAS.
- B. J. Gurley, S. F. Gardner, M. A. Hubbard, D. K. Williams, W. B. Gentry, Y. Cui and C. Y. W. Ang, Drugs Aging, 2005, 22, 525–539 Search PubMed.
- J. S. Markowitz, J. L. Donovan, C. L. DeVane, R. M. Taylor, Y. Ruan, J. S. Wang and K. D. Chavin, JAMA, J. Am. Med. Assoc., 2003, 290, 1500–1504 Search PubMed.
- R. H. Mathijssen, J. Verweij, P. de Bruijn, W. J. Loos and A. Sparreboom, J. Natl. Cancer Inst., 2002, 94, 1247–1249 Search PubMed.
- S. C. Piscitelli, A. H. Burstein, D. Chaitt, R. M. Alfaro and J. Falloon, Lancet, 2000, 355, 547–548 Search PubMed.
- Public Health Advisory, FDA, February 10, 2000, URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm052238.htm Search PubMed.
- L. Chen, M. J. Lee, H. Li and C. S. Yang, Drug Metab. Dispos., 1997, 25, 1045–1050 CAS.
- W. D. Johnson, R. L. Morrissey, J. A. Crowell and D. L. McCormick, Toxicologist, 1999, 48, 57–58 Search PubMed.
- W. Alhusainy, A. Paini, A. Punt, J. Louisse, A. Spenkelink, J. Vervoort, T. Delatour, G. Scholz, B. Schilter, T. Adams, P. J. van Bladeren and I. M. C. M. Rietjens, Toxicol. Appl. Pharmacol., 2010, 245, 179–190 Search PubMed.
- S. M. F. Jeurissen, A. Punt, T. Delatour and I. M. C. M. Rietjens, Food Chem. Toxicol., 2008, 46, 2296–2302 Search PubMed.
Footnote |
| † This paper forms part of the themed issue on Plant Food Supplements: Regulatory, scientific and technical issues concerning safety, quality and efficacy. |
| This journal is © The Royal Society of Chemistry 2011 |