Antioxidant and antihepatotoxic effect of Spirulina laxissima against carbon tetrachloride induced hepatotoxicity in rats
Gini C.
Kuriakose†
*a and
Muraleedhara G.
Kurup
*b
aDepartment of Applied Biochemistry, Mahatma Gandhi University, Kottayam, Kerala, India. E-mail: ginibesant@gmail.com
bDepartment of Biochemistry, Kerala University, Palayam Campus, Thiruvananthapuram, 695035, Kerala, India. E-mail: muraleedharakurup@gmail.com; Tel: +91 471 2443456
First published on 1st February 2011
Abstract
The vast biodiversity of nature provides bioactive compounds that may be useful in the fight against chronic diseases. This study was designed to investigate the protective effects of the ethanol extract of Spirulina laxissima West (Pseudanabaenaceae) (EESL) against carbon tetrachloride (CCl4) induced hepatotoxicities in rats. Male albino rats of Sprague-Dawley strain were treated orally with the ethanol extract of S. laxissima (50, 100 mg kg−1 body wt.) 1 h before each CCl4 administration. The ethanol extract of S. laxissima showed the maximum antioxidant property in vitro. There were statistically significant losses in the activities of antioxidant enzymes and an increase in TBARS and liver function marker enzymes in the serum of the CCl4-treated group compared with the control group. However, all the tested groups were able to counteract these effects. The antioxidant activity of the extracts might be attributable to its proton-donating ability, as evidenced by DPPH. In the present study, the decline in the level of antioxidant observed in CCl4-treated rats is a clear manifestation of excessive formation of radicals and activation of the lipid peroxidation system resulting in tissue damage. The significant increases in the concentration of antioxidant enzymes in tissues of animals treated with CCl4 + EESL indicate the antioxidant effect of EESL. This study suggests that EESL can protect the liver against CCl4-induced oxidative damage in rats, and the hepatoprotective effect might be correlated with its antioxidant and radical-scavenging effects.
Introduction
Liver disease remains a serious health problem, and is caused by drugs, chemicals, and alcohol. The liver plays an essential role in metabolism of drugs and xenobiotics, and in maintaining biological equilibrium. The role played by this organ in the removal of substances from the portal circulation makes it the first in line to be subject to attack by foreign materials. Despite the tremendous strides in modern medicine, there is hardly any drug that stimulates liver function, offers protection to the liver from damage, or helps regeneration of hepatic cells.1 Natural drugs are frequently considered to be less toxic and free from side-effects than synthetic drugs. The search for crude drugs of plant origin with antioxidant activity has become a central focus for study of hepatoprotection today.Focusing our attention on natural and bioavailable sources of antioxidants, we undertook to investigate the antioxidant properties of the cyanophyte Spirulina laxissima West (Pseudanabaenaceae), a unicellular blue-green alga and that is consumed as a nutrient-dense food source and for its health-enhancing properties. Spirulina is an important source of the blue photosynthetic pigment phycocyanin (PC), which has been described as a strong antioxidant2 and anti-inflammatory3 natural compound, as evidenced by in vitro and in vivo studies on PC from the cyanophyte Spirulina platensis Geitler (Pseudanabaenaceae). PC is a water-soluble phycobiliprotein composed of a and h subunit polypeptides which associate into ah monomers4 in turn, have a high affinity to assemble together to form (ah)3 trimers and finally (ah)6 hexamers. The a and h subunits are constituted of a protein backbone to which linear tetrapyrrole chromophores are covalently bound.5 The chromophore, named phycocyanobilin, is similar in chemical structure to bilirubin, and like the latter acts as a powerful scavenger of reactive oxygen species.6
Carbon tetrachloride (CCl4), a potent hepatotoxic agent, is biotransformed to a trichloromethyl radical by the cytochrome P450 system in liver microsomes, and consequently causes lipid peroxidation of membranes that leads to liver injury.7 The present study was designed to evaluate the putative antioxidant action of ethanol extract of S. laxissima in an experimental model of CCl4-induced hepatotoxicity in albino rats.
Materials and methods
Cyanobacteria
The cyanobacterial strain Spirulina laxissima West (Family: Pseudanabaenaceae, Order: Oscillatoriales) procured from NCCUBGA, IARI, New Delhi, India, was used for the experiments. A stock culture of the strain was maintained in our laboratory by using BG-11 medium.8Cyanobacteria (50 ml) from a mid-log-phase growth culture was dispersed aseptically into cotton-plugged 1000 ml sterilized conical flasks. These were maintained at 25 ± 2 °C under 24 h light in an illuminated chamber at 2.5 kilolux.Preparation of the extract of S. laxissima
Extraction from S. laxissima was carried out according to the procedure of Ferrigni.9 The cyanobacterial culture was grown up to the mid-log phase (10–14 days old) and then it was harvested by filtration, removing the medium through a coarse filter paper. Then the harvested cyanobacterial mass was washed twice with distilled water to completely remove the culture medium. Approximately 8–10 ml water was used for every 1 g culture harvested. The harvested S. laxissima was dried at 45–50 °C for 48 h. The dried material took the form of granules, and this was macerated in liquid nitrogen in a pestle and mortar until a fine powder was obtained. The powdered material was mixed with petroleum ether and sonicated to break open the cell walls (25 g powder was mixed with 62.5 ml of solvent). Then it was placed on a shaker platform for 24 h for cold extraction. The filtrate was collected by centrifugation and the material obtained was repeatedly cold-extracted with solvents of increasing polarity (chloroform, ethanol, methanol and distilled water). The filtrate was evaporated by rotary evaporator at temperature 30–35 °C and the mass obtained was dissolved in distilled water and employed for further experiments. The cold-extraction procedure was repeated four times. The extracts were preserved in vials at −20 °C. A maximum dose of 100 mg of extract per kg body weight was used for treatment in experiments. Higher doses such as 250 mg per kg body weight showed almost the same results (data not given), so the lower concentration was selected for the study.Experimental animals
Male albino rats of the Sprague-Dawley strain weighing between 120–150 g were purchased from the Small Animal Breeding Section of Kerala Agriculture University, Mannuthy, Trichur, Kerala, India. The animals were maintained in an animal house with standard facilities having CPCEA approval (No. 732). The animals were housed in polypropylene cages and maintained at 25 ± 2 °C under 12 h light–dark cycle. They were fed with Amrut Laboratory Animal Feed, manufactured by Nav, Maharashtra Chakan Oil Mills Ltd, Pune. Water was provided ad libitum. The animals were acclimatized for one week under laboratory conditions. All the pharmacological experimental protocols were approved by the institutional animal ethics committee (Mahatma Gandhi University, Kerala, India).DPPH radical-scavenging activity
Reduction of 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH) to diphenylpicryl hydrazine by EESL was measured spectrophotometrically at 517 nm.10L-Ascorbic acid was used as a reference compound, and data were expressed as the percent decrease in the absorbance compared to the control. The optical density was recorded and % inhibition was calculated using the formula given below| Inhibition of DPPH activity = [(A − B)/A] × 100% |
Experimental design
Hepatoprotective activity against the CCl4-induced chronic toxicity was determined by the method of Jose and Kuttan.11 The animals were divided into five groups of eight animals each and treated as follows:Group I – Vehicle
Group II – CCl4 in paraffin oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, 1.5 ml kg−1 body weight, i.p.)
5 v/v, 1.5 ml kg−1 body weight, i.p.)
Group III – Ethanol extract (oral) of S. laxissima (50 mg kg−1 body weight) + CCl4 in paraffin oil
Group IV – Ethanol extract (oral) of S. laxissima (100 mg kg−1 body weight) + CCl4 in paraffin oil
Group V – Silymarin (oral) (100 mg kg−1 body weight) + CCl4 in paraffin oil
Animals in Groups II, III and IV and V were treated with CCl4 three times a week for five weeks (total 15 doses). Groups III, IV and V were treated orally with ethanol extract of S. laxissima (50, 100 mg) and silymarin respectively one hour before each CCl4 administration. The group treated with the vehicle was kept as normal. Group II, treated with CCl4 alone, was kept as an untreated control. Twenty-four hours after the last dose of CCl4, the animals were sacrificed by decapitation, and the blood was collected by cutting the jugular vein. A portion of the liver was used for histopathological analysis. The liver in each case was dissected out, the blood blotted off, and washed in cold saline. The serum was separated from the blood, and the serum and liver samples were stored at −80 °C until analysis.
Determination of the levels of liver function marker enzymes in serum
The level of alanine aminotransferase and aspartate aminotransferase,12γ-glutamyl transpeptidase13 and alkaline phosphatase14 were measured using a standard assay procedure.Preparation of liver supernates
Prior to biochemical analysis, each liver sample (100 mg ml−1buffer) was homogenized in 50 mM phosphate buffer (pH 7.0); the homogenate was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and the supernatant obtained was used for biochemical analysis. All liver parameters were expressed as activity per mg protein. The protein concentration in each fraction was determined by the method of Lowry15 using crystalline bovine serum albumin as a standard.
000 rpm for 15 min and the supernatant obtained was used for biochemical analysis. All liver parameters were expressed as activity per mg protein. The protein concentration in each fraction was determined by the method of Lowry15 using crystalline bovine serum albumin as a standard.
Determination of lipid peroxidation
The mean thiobarbituric acid reactive substances (TBARS) (μmol mg−1protein), a measure of lipid peroxidation, was assayed in the form of thiobarbituric acid reacting substances by the method of Nichans and Samuelson,16 and conjugate dienes (CDs) were assayed by the method of Beuje and Aust.17Quantitative analysis of enzyme activities
Activities of superoxide dismutase,18catalase,19 reduced glutathione,20glutathione peroxidase,21 and glutathione transferase22 were assayed by the standard protocols.Determination of protein and bilirubin
The protein content (g per 100 ml serum) and bilirubin (mg per 100 ml serum) content in the serum were evaluated by using the standard procedure of Lowry15 and Malloy & Evelyn23 respectively.Lipid profile of liver
The concentrations of total lipids,24phospholipids,25triglycerides26 and cholesterol27 were measured in liver (mg per 100 g tissue) by the standard procedures.Histopathological examination of liver
Conventional techniques of paraffin wax sectioning and haematoxylin–eosin staining were used for histological studies. Slices of fresh liver tissue were cut and fixed in buffered neutral formalin fixative for 24 h. Following fixation, the tissues were washed and processed through an ascending series of alcohol solutions (30, 50, 70, 90 and 100%), cleared in methyl salicylate and infiltrated with wax at 57 °C. The tissues thus cleared were embedded in paraffin. Sections of 6–8 μm thickness were cut, stained by aqueous haematoxylin and alcoholic eosin, and examined by bright-field microscopy at a magnification of 400×.Statistical analysis
The results are presented as the mean of 8 animals in each group ± SD. The data were obtained by one-way ANOVA followed by Dunnett’s t-test. The level of significance was set at P < 0.01.Results
The antioxidant activity of different extracts of S. laxissima was evaluated by their DPPH radical-scavenging capacity. Fig. 1 shows the percent of DPPH radical-scavenging capacity with L-ascorbic acid as a reference. The experimental data reveal that all of these extracts at various levels are likely to have radical-scavenging capacity. From Fig. 1, we observe that a dose–response relationship is found; the activity increased as the concentration increased for each extract. Among the three extracts used in the experiment, 30 μg mL−1 of ethanol extract was the strongest, with 77.78%, then 30 μg mL−1 of methanol extract with 65.09% and 30 μg mL−1 of water extract with 62.39% DPPH radical-scavenging activity. The percent inhibition of DPPH radical-scavenging activity of L-ascorbic acid was found to be 84%.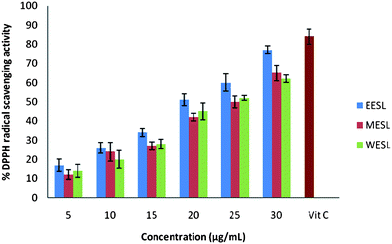 | ||
| Fig. 1 Percent DPPH radical-scavenging capacity of different extracts of S. laxissima (ethanol extract (EESL), methanol extract (MESF) and water extract (WESF)). | ||
A significant increase in the activity of the serum enzymes AST, ALT, GGT and ALP was observed in rats receiving CCl4 in the vehicle (Group II) when compared to normal (Group I) rats administered with the vehicle alone (Table 1). However, the activities of these serum enzymes were significantly (P < 0.01) lower in rats treated with the EESL (Group III and IV) than in Group II rats. The protection offered by silymarin was found to be higher.
| Group | AST (U/L serum) | ALT (U/L serum) | ALP (U/L serum) | GGT (U/L serum) |
|---|---|---|---|---|
| a Values are mean ± SD of 8 animals in each group. Statistical analysis: ANOVA followed by Dunnett’s t-test. P < 0.01 as compared with Group I (*), Group II (†). | ||||
| I – Normal | 38.36 ± 7.09 | 29.60 ± 8.35 | 89.61 ± 12.98 | 3.94 ± 0.96 |
| II – CCl4 + LP | 137.61 ± 11.54* | 178.38 ± 9.09* | 196.92 ± 13.92* | 9.64 ± 0.34* |
| III – CCl4 + EESL (50 mg) | 90.32 ± 10.58† | 86.19 ± 12.45† | 114.69 ± 7.87† | 7.34 ± 1.87† |
| IV – CCl4 + EESL (100 mg) | 43.44 ± 12.94† | 34.15 ± 9.22† | 93.97 ± 9.16† | 4.29 ± 1.24† |
| V – CCl4 + silymarin | 39.38 ± 11.24† | 30.17 ± 10.68† | 91.62 ± 6.83† | 3.90 ± 0.08† |
A marked increase in the mean TBARS and CD level was found in the liver of Group II (CCl4-exposed) rats relative to normal (Group I) rats (Table 2); this increase was statistically significant (P < 0.01). Treatment with EESL in Group IV rats was found to result in a significant (P < 0.01) lowering of the mean TBARS and CD concentration, presumably by limiting lipid peroxidation in the hepatic tissue. CCl4 administration in Group II rats resulted in a marked decrease (relative to normal) in the level of reduced glutathione in the liver (Table 2); this decrease was statistically significant (P < 0.01). Treatment with EESL and silymarin resulted in a significantly higher concentration of GSH (P < 0.01) than that in Group II.
| Group | TBARS (mM per 100 g tissue) | CD (mM per 100 g tissue) | GSH (mM per 100 g tissue) |
|---|---|---|---|
| a Values are mean ± SD of 8 animals in each group. Statistical analysis: ANOVA followed by Dunnett’s t-test. P < 0.01 as compared with Group I (*), Group II (†). | |||
| I – Normal | 1.39 ± 0.65 | 62.24 ± 10.19 | 0.94 ± 0.02 |
| II – CCl4 + LP | 1.68 ± 0.56* | 80.40 ± 15.54* | 0.45 ± 0.04* |
| III – CCl4 + EESL (50 mg) | 1.53 ± 0.87† | 67.14 ± 12.53† | 0.76 ± 0.14† |
| IV – CCl4 + EESL (100 mg) | 1.43 ± 0.66† | 64.02 ± 9.46† | 0.88 ± 3.29† |
| V – CCl4 + silymarin | 1.40 ± 0.17† | 62.56 ± 7.07† | 0.90 ± 0.16† |
The concentrations of total proteins and bilirubin in serum are given in the Table 3. A marked elevation in the concentration of bilirubin and decreases in protein content was observed in the CCl4-treated rats compared to normal animals (P < 0.01). In rats that received EESL at a dose of 100 mg kg−1 body weight, the concentrations of bilirubin and total protein were maintained at near-normal levels.
| Group | Protein (g per 100 ml serum) | Bilirubin (mg per 100 ml serum) |
|---|---|---|
| a Values are mean ± SD of 8 animals in each group. Statistical analysis: ANOVA followed by Dunnett’s t-test. P < 0.01 as compared with Group I (*), Group II (†). b c | ||
| I – Normal | 8.65 ± 2.56 | 1.71 ± 0.09 |
| II – CCl4 + LP | 6.98 ± 1.15* | 2.48 ± 0.14* |
| III – CCl4 + EESL (50 mg) | 7.11 ± 1.87† | 2.02 ± 0.54† |
| IV – CCl4 +EESL (100 mg) | 8.55 ± 1.32† | 1.79 ± 0.18† |
| V – CCl4 + silymarin | 8.59 ± 0.16† | 1.74 ± 0.20† |
Table 4 shows the concentrations of total lipids, phospholipids, cholesterol, and triglycerides in the liver. Significant increase (P < 0.01) in the lipid profile was observed in CCl4-intoxicated rats. Co-administration of EESL significantly prevented the CCl4-induced alterations in the lipid profile.
| Group | Total lipids (mg per 100 g tissue) | Phospholipids (mg per 100 g tissue) | Cholesterol (mg per 100 g tissue) | Triglycerides (mg per 100 g tissue) |
|---|---|---|---|---|
| a Values are mean ± SD of 8 animals in each group. Statistical analysis ANOVA followed by Dunnett t-test. P < 0.01 as compared with Group I (*), Group II (†). | ||||
| I – Normal | 4375.88 ± 12.01 | 2254.64 ± 9.01 | 565.35 ± 3.16 | 447.44 ± 9.24 |
| II – CCl4 + LP | 5685.72 ± 15.29* | 3017.41 ± 13.01* | 784.92 ± 4.24* | 561.23 ± 6.96* |
| III – CCl4 + EESL (50 mg) | 5259.89 ± 13.52† | 2765.26 ± 11.45† | 654.36 ± 10.45† | 470.96 ± 9.63† |
| IV – CCl4 + EESL (100 mg) | 4797.71 ± 9.73† | 2420.44 ± 10.12† | 571.80 ± 11.25† | 456.64 ± 14.34† |
| V – CCl4 + silymarin | 4590.45 ± 12.44† | 2311.69 ± 13.58† | 569.76 ± 12.38† | 451.82 ± 10.67† |
A significant decrease in antioxidant enzymes like CAT, SOD, GPX and GST activity was observed in the liver of CCl4-administered (Group II) rats when compared to normal (Group I) rats that had received the vehicle alone (Table 5). Treatment with the EESL appeared to exert a beneficial effect, since the activities of these enzymes were significantly (P < 0.01) higher in the livers of Group III and IV rats than in Group II rats.
| Group | SOD (U per mg protein) | CAT (U per mg protein) | GPX (U per mg protein) | GST (U per mg protein) |
|---|---|---|---|---|
| a Values are mean ± SD of 8 animals in each group. Statistical analysis ANOVA followed by Dunnett’s t-test. P < 0.01 as compared with Group I (*), Group II (†). | ||||
| I – Normal | 5.33 ± 1.03 | 2.93 ± 0.09 | 165.96 ± 10.12 | 0.34 ± 0.17 |
| II – CCl4 + LP | 4.14 ± 1.49* | 0.54 ± 0.38* | 142.12 ± 7.09* | 0.24 ± 0.04* |
| III – CCl4 + EESL 50 mg | 4.48 ± 1.78† | 1.99 ± 0.69† | 155.49 ± 12.09† | 0.27 ± 0.19† |
| IV – CCl4 + EESL 100 mg | 5.16 ± 0.41† | 2.78 ± 0.17† | 163.95 ± 11.43† | 0.31 ± 0.04† |
| V – CCl4 + silymarin | 5.30 ± 1.19† | 2.80 ± 0.05† | 164.84 ± 5.95† | 0.33 ± 0.09† |
When compared to the histoarchitecture of the livers of Group I (normal) animals (Fig. 2A), liver cells of Group II rats (exposed to CCl4) revealed extensive damage, characterized by the disruption of the lattice nature of the hepatocyte, damaged cell membranes, degenerated nuclei, a disintegrated central vein and damaged hepatic sinusoids (Fig. 2B). In Group IV rats (exposed to CCl4 + EESL (100 mg)), only minimal disruption of the hepatic cellular structure was observed (Fig. 2C). Liver section of rats treated with CCl4 and silymarin (Group V) showed minimal inflammatory cellular infiltration (Fig. 2D).
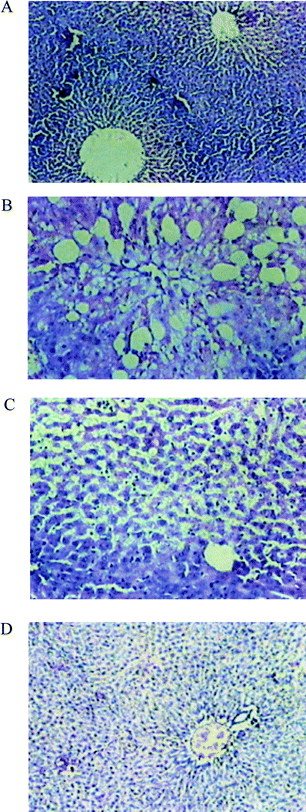 | ||
| Fig. 2 Photomicrographs of liver sections of rats stained with haematoxylin and eosin (200X). A: Normal rat showing a central vein surrounded by normal hepatocytes. B: CCl4-treated rats showing a dilated central vein and hepatocytes with fatty change and ballooning degeneration. C: CCl4 + S. laxissima (100 mg kg−1 body weight) treated rats showing the central vein and normal hepatocytes with occasional hepatocytes showing fatty change and ballooning degeneration. D: Liver section of rats treated with CCl4 and silymarin showing minimal inflammatory cellular infiltration, and near-normal liver architecture. | ||
Discussion
Phycocyanin (PC) is a water-soluble, highly fluorescent protein derived from cyanobacteria (blue-green algae) used in food coloring,28 cosmetics29 and biomedical research.30 Recently, it has been demonstrated that PC from Spirulina platensis has significant antioxidant and radical-scavenging properties both in vivo and in vitro models,31 and is a potential therapeutic agent in diseases induced by oxidative stress. In this work, we focused our attention on the edible microalga S. laxissima, in which PC represents around 15% of dry weight. In particular, we tested the efficacy of different extracts of S. laxissima against the oxidative damage induced by DPPH. As compared with the commercial antioxidants, the ethanol extract of S. laxissima showed comparable scavenging activity on DPPH. It was, however, evident that extracts of S. laxissima did show antioxidant activityin vitro. The antioxidant activity of the extracts might be attributed to its proton-donating ability, as evidenced through DPPH.Spirulina species have been used as food for thousands of years. Among blue-green algae, many species have documented biomodulatory effects. Many medicinal properties have been attributed to Spirulina species, including reduction in body weight,32reduction of cholesterol,33 increased activity of lipase,34reduction of glucose levels,35 modulation of carcinogen metabolic enzymes,36 modulation of lead toxicity37 and radical-scavenging action.38
Carbon tetrachloride (CCl4) is a well-known hepatotoxic agent. The changes associated with CCl4-induced liver damage are similar to those of acute viral hepatitis.39 CCl4-induced liver damage is a classic model used for the screening of hepatoprotective drugs. The basis of its hepatotoxicity lies in its biotransformation by the cytochrome P450 system to two radicals. The first metabolite, a trichloromethyl radical, forms covalent adducts with lipids and proteins; it can interact with O2 to form a second metabolite, a trichloromethyl peroxyl radical, or can remove hydrogen atoms to form chloroform. This sequence of events leads to lipid peroxidation of membranes and consequent liver injury. In response to this hepatocellular injury, “activated” hepatic Kupfer cells release increased quantities of active oxygen species and other bioactive agents.40
Since radicals play such an important role in CCl4-induced hepatotoxicity, it seems logical that compounds that neutralize such radicals may have a hepatoprotective effect. Indeed, various natural products have been reported to protect against CCl4-induced hepatotoxicity.41 Ample experimental and epidemiological studies support the involvement of oxidative stress in the pathogenesis and progression of several chronic diseases. In the present study it was found that EESL could effectively scavenge the radicals in a dose-dependent manner. It has been reported that CCl4 caused significant increase in hepatic lipid peroxidation due to radical injury in cirrhotic livers of rats.42 In the present study, elevated levels of TBARS and CD observed in CCl4-treated rats indicate excessive formation of radicals and activation of the lipid peroxidation system, resulting in hepatic damage. The significant decline in the concentration of these constituents in the livers of rats treated with CCl4 + EESL indicates the anti-lipid-peroxidative effect of S. laxissima.
The antioxidant properties of Nostoc sphaeroides Kützing (Nostocaceae) and Aulosira fertilisima Ghose (Nostocaceae) on CCl4-induced hepatic damage in rats had been reported earlier from our laboratory.43 It has been reported that C-phycocyanin from Spirulina platensis effectively inhibited CCl4-induced lipid peroxidation in rat liver in vivo.44GSH is a major non-protein thiol in living organisms which plays a central role in coordinating the body's antioxidant defense processes.45 Decline in the GSH content in the liver of CCl4-intoxicated rats, and its subsequent return towards the near-normality in the group administered with CCl4 + EESL, also reveal the anti-lipid-peroxidative effect of S. laxissima.
Estimating the activities of serum marker enzymes such as AST, ALT, ALP and GGT, can enable assessment of liver function. When the liver cell plasma membrane is damaged, a variety of enzymes normally located in the cytosol are released into the bloodstream. Their amount in the serum is a useful quantitative marker of the extent and type of hepatocellular damage.46 The normalization of the above enzyme levels seen in rats treated with the cyanobacterial formulation (100 mg kg−1 body weight) indicates the possibility of EESL being able to inhibit liver cell injury and reducing the leakage of the above enzymes in to the blood. It has been reported that serum transaminases return to normal levels with the healing of liver parenchyma and regeneration of liver cells.47 It was also reported that the alcohol extract of Spirulina maxima Geitler (Pseudanabaenaceae) inhibited lipid peroxidation more significantly than the chemical antioxidants like α-tocopherol and β-carotene.48Phycocyanin significantly reduced the hepatotoxicity caused by CCl4, which induces the formation of radicals. The hepatoprotective effect of phycocyanin was therefore attributed to the inhibition of reactions involved in the formation of reactive metabolites, and possibly due to its radical-scavenging activity.49
The site-specific oxidative damage of some of the susceptible amino acids of protein is now regarded as the major cause of metabolic dysfunction during pathogenesis. The capacity of liver synthesize albumins is adversely affected by hepatotoxins.50 Administration of hepatotoxins like CCl4 causes depression in protein biosynthesis, which is due to the disruption and disassociation of polyribosomes from the endoplasmic reticulum. The lowered levels of total proteins recorded in the serum of CCl4-treated rats can be attributed to these features. Attainment of near-normality in protein content of serum in rats treated with CCl4 + LP + EESL further confirmed the anti-hepatotoxic effect of S. laxissima.
The body has an effective mechanism to prevent and neutralize radical-induced damage. This is accomplished by a set of endogenous antioxidant enzymes, such as SOD, CAT, GPX and GST. When the balance between ROS production and antioxidant defenses is lost, oxidative stress results, which through a series of events deregulates the cellular functions, leading to various pathological conditions.51 Any compound, natural or synthetic, with antioxidant properties may contribute towards the partial or total alleviation of this type of damage. In the present study, decline in the level of antioxidant enzymes like SOD, CAT, GPX and GST observed in CCl4-treated rats is a clear manifestation of the excessive formation of radicals and activation of lipid peroxidation system, resulting in tissue damage. The significant increases in the concentration of these constituents in liver tissues of animals treated with CCl4 + EESL indicate the antioxidant effect of EESL. It was reported that Dunaliella salina Teodoresco (Dunaliellaceae), a green marine alga, has the ability to protect against oxidative stress in vivo using animal models.52 It has been established that carotenoids from microalgae exert their action against CCl4 liver injury by lipid peroxidation, either through decreased production of radical derivatives or due to the antioxidant activity of the protective agent itself.53 The antioxidant activity of Laminaria japonica Areschoug (Laminariaceae) and Ecklonia stolonifera Okamura (Lessoniaceae) against CCl4-induced hepatotoxicity has been reported.54
During attack by toxins, the lipid profile of serum and tissues increases. CCl4-poisoned rats appear to have a deranged hepatic triglyceride secretory mechanism. Accumulation of triglycerides in the liver during CCl4 poisoning results not from a defect in the release of triglycerides into plasma, but perhaps from an increase in hepatic synthesis of triglycerides.55 Treatment of rats with CCl4 induces centrilobular necrosis, which results in the accumulation of fat in the liver. Fat from the peripheral adipose tissue is translocated to the liver and kidney, leading to its accumulation during toxicity. CCl4 also interferes with hepatic phospholipid synthesis. Intoxication of experimental animals with CCl4 altered the membrane structure and function, as shown by the increases in cholesterol and phospholipid concentrations, and hence an increased cholesterol-to-phospholipid ratio.56 Pre-treatment of experimental animals with EESL extract prevented the alterations of membrane fluidity, with a decrease in the cholesterol-to-phospholipid ratio, which was elevated by animals treated just with CCl4 alone. Thus S. laxissima plays a role in peroxidation by inhibiting the radical attack on biomembranes. It has been reported that C-phycocyanin from Spirulina platensis effectively inhibits marked CCl4-induced changes in the lipid profile of the rat liver.57
Conclusion
The present study demonstrates the hepatoprotective and antioxidant properties of the ethanol extract of S. laxissima (EESL). The hepatoprotective effect of EESL may be due to the presence of phycocyanin pigment. In this regard, it is relevant to point out that phycocyanins have been suggested to act as antioxidants and exert their antioxidant activity by scavenging lipid peroxidation. Thus a plausible mechanism of the hepatoprotective effect of EESL may be due to its antioxidant effect. Further studies are needed to identify and isolate the active principles of EESL, which could offer antioxidant and hepatoprotective properties.Acknowledgements
We owe our thanks to Professor Ramesh, Department of Statistics, Rubber Research Institute, Kottayam, India, for giving valuable support for statistical analysis. Financial assistance from the University Grant Commission, India, is acknowledged.References
- E. Porchezhian and S. H. Ansari, Phytomedicine, 2005, 12, 62–64 CrossRef CAS.
- V. B. Bhat and K. M. Madyastha, Biochem. Biophys. Res. Commun., 2000, 275, 20–25 CrossRef CAS.
- C. M. Reddy, V. B. Bhat, G. Kiranmai, M. N. Reddy, P. Reddanna and K. M. Madyastha, Biochem. Biophys. Res. Commun., 2000, 277, 599–603 CrossRef CAS.
- A. N. Glazer, Methods Enzymol., 1988, 167, 291–303 Search PubMed.
- A. K. Padyana, V. B. Bhat, K. M. Madyastha, K. R. Rajashankar and S. Ramakumar, Biochem. Biophys. Res. Commun., 2001, 282, 893–898 CrossRef CAS.
- R. Stocker, A. F. McDonagh, A. N. Glazer and B. N. Ames, Methods Enzymol., 1990, 186, 301–309 CAS.
- R. O. Recknagel, E. A. Glende, J. A. Dolak and R. L. Waller, Pharmacol. Ther., 1989, 43, 139–154 CrossRef CAS.
- R. Y. Stainer, R. Kunisawa, M. Mandel and B. Cohen, Bacteriol. Rev., 1971, 5, 171–176.
- N. R. Ferrigni, J. E. Putnam, B. Anderson and L. B. Jacobson, J. Nat. Prod., 1982, 45, 679–686 CrossRef CAS.
- A. K. Ratty, J. Sunamoto and N. P. Das, Biochem. Pharmacol., 1988, 37, 989–995 CrossRef CAS.
- J. K. Jose and R. Kuttan, J. Ethnopharmacol., 2000, 72, 135–140 CrossRef CAS.
- A. F. Mohum and J. Y. Cook, J. Clin. Pathol., 1957, 10, 394–398 CrossRef.
- A. H. Gowenlock, Varley's Practical Clinical Biochemistry, London, United Kingdom, 1988, pp. 518–526 Search PubMed.
- H. Varley, Practical Clinical Biochemistry, CBS, New Delhi, India, 1988, pp. 453–457 Search PubMed.
- O. H. Lowry, N. J. Roesbrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–269 CAS.
- W. G. Nichans and B. Samuelson, Eur. J. Biochem., 1968, 6, 126–133 CrossRef.
- J. A. Beuje and S. D. Aust, Antioxidants, in Methods in Enzymology, ed. S. P. Clowick and N. O. Kaplan, Academic Press, New York, 1978, pp. 302–305 Search PubMed.
- P. Kakkar, B. Das and D. N. Vishwanath, Indian J. Biochem. Biophys., 1984, 21, 130–138 Search PubMed.
- A. L. Calibrone, Handbook of Methods for Oxygen Radical Research, Florida, USA, 1985, pp. 283–287 Search PubMed.
- E. Bentler and B. M. Kelly, Experientia, 1963, 19, 96–101 CrossRef.
- J. R. Rotruck, A. Pope and H. E. Ganther, Science, 1973, 17, 486–490.
- E. Beutler, Red Cell Metabolism: A Manual of Biochemical Methods, Gruene FP Stratton, New York, 1986, pp. 57–59 Search PubMed.
- H. J. Malloy and K. A. Evelyn, J. Biol. Chem., 1937, 119, 481–496 CAS.
- J. Folch, M. Lees and S. G. Solane, J Biol Chem., 1957, 226, 497–509 CAS.
- H. V. Connerty, A. R. Briggs and E. H Eaton, Clin. Chem., 1961, 7, 37–53 CAS.
- E. V. Handel and D. B. Zilversmith, Serum triglycerides micro method, in Gradwohl's clinical laboratory methods and diagnosis, ed. S. Frankel, S. Reitman and A. C. Sonnenwirth, The Mosby Co., Saint Louis, USA, 1963pp. 263–267 Search PubMed.
- A. Zlatkis, B. Zak and G. J. Boyle, J. Lab. Clin. Med., 1953, 41, 486–494 CAS.
- A. Yoshida, Y. Takagaki and T. Nishimune, Biosci., Biotechnol., Biochem., 1996, 60, 57–60 CrossRef CAS.
- Z. Cohen, Products from microalgae, in Handbook of Microalgal Mass Culture, ed. A. Richmond, CRC Press, Boca Raton, USA, 1986, pp. 421–425 Search PubMed.
- A. N. Glazer and L. Stryer, Methods Enzymol., 1990, 184, 188–194 CAS.
- C. Romay and R. Gonzalez, J. Pharm. Pharmacol., 2000, 52, 367–372 CrossRef CAS.
- E. Becker and B. Jakover, Nutr. Rep. Int., 1986, 33, 565–574 Search PubMed.
- K. G. Hori, G. Ishibashi and M. H. Luan, Plant Foods Hum. Nutr., 1994, 45, 63–70 CrossRef CAS.
- K. Iwata and T. Inayama, J. Nutr. Sci. Vitaminol., 1999, 36, 165–171 Search PubMed.
- Y. Takai and Y. Hosoyamada, J. Jpn. Soc. Nutr. Food Sci., 1991, 44, 273–277 Search PubMed.
- A. Mittal and P. V. Kumar, Phytother. Res., 1999, 13, 111–114 CrossRef CAS.
- D. Shastri, M. Kumar and A. Kumar, Phytother. Res., 1999, 13, 258–260 CrossRef CAS.
- V. B. Bhat and K. M. Madyastha, Biochem. Biophys. Res. Commun., 2001, 285, 262–266 CrossRef CAS.
- A. E. Eisisi, D. L. Earnest and I. G. Sipes, Toxicol. Appl. Pharmacol., 2003, 119, 295–301.
- G. Hsiao, M. Y. Shen, K. H. Lin, M. H. Lan and J. R. Sheu, J. Agric. Food Chem., 2004, 51, 3302–3308.
- A. S. Girish, W. G. Sudhir and D. K. Avinash, J. Ethnopharmacol., 2004, 90, 229–235 CrossRef.
- K. Vivek, K. K. Pillai, S. Z. Hussain and D. K. Balani, Indian J. Pharmacol., 1994, 26, 26–31 Search PubMed.
- K. C. Gini and K. G. Muraleedhara, Indian J. Exp. Biol., 2008, 46, 52–56 Search PubMed.
- B. Serena, R. Sara, B. Francesca, F. Sonia and P. Silvia, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2006, 833, 12–17 CrossRef CAS.
- R. Ferrari, C. Ceconi, S. Curello and A. Cargnoni, Cardiovasc. Pharmacol. Ther., 1991, 52, 77–82 Search PubMed.
- K.-T. Ha, S.-J. Yoon, D.-Y. Choi and D.-W. Kim, J. Ethnopharmacol., 2005, 96, 529–534 CrossRef.
- T. Rajmohan and L. L. Anthony, Indian J. Biochem. Biophys., 2003, 40, 354–359 Search PubMed.
- M. S. Miranda, R. G. Cintra, S. M. Barros and J. Mancini-Filho, Braz. J. Med. Biol. Res., 1998, 31, 1075–1081 Search PubMed.
- B. B. Vadiraja, N. W. Gaikwad and K. M. Madyastha, Biochem. Biophys. Res. Commun., 1998, 249, 428–431 CrossRef CAS.
- B. Uday, D. Dipak, E. Banerjee and K. Ranjith, Curr. Sci., 1999, 5, 658–662.
- J. A. Castro, G. C. Ferrya, C. R. Castro and J. R. Gillette, Biochem. Pharmacol., 1974, 23, 295–301 CrossRef CAS.
- K. N. Chidambara, J. Rajesha, S. M. Mahadeva and G. A. Ravishankar, J. Med. Food, 2005, 8, 523–527 CrossRef.
- C. Kerfeld, Photosynth. Res., 2004, 81, 215–219 CrossRef CAS.
- X. Zhao, C.-H. Xue, Z.-J. Li, Y.-P. Cai and H.-Y. Liu, J. Appl. Phycol., 2004, 16, 111–115 Search PubMed.
- N. F. Kushnerobn, S. E. Fomenko, M. I. Polozhentseva and A. E. Bulanov, Vopr. Med. Khim., 1995, 41, 20–23 Search PubMed.
- R. A. Cooper, J. R. Durocher and M. H. Leslie, J. Clin. Invest., 1997, 60, 115–21.
- J. E. Pinero, B. P. Bermejo and A. M. Villardel, Il Pharmaco., 2001, 56, 497–503 Search PubMed.
Footnote |
| † Present address: Department of Biochemistry, Indian Institute of Science, Bangalore, 560012, India. |
| This journal is © The Royal Society of Chemistry 2011 |
