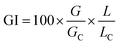Distribution and availability of trace elements in municipal solid waste composts
Remigio
Paradelo
*a,
Antía
Villada
a,
Rosa
Devesa-Rey
a,
Ana Belén
Moldes
b,
Marta
Domínguez
c,
Jacobo
Patiño
c and
María Teresa
Barral
a
aDepartamento de Edafoloxía e Química Agrícola, Facultade de Farmacia, Universidade de Santiago de Compostela, Campus Sur s/n, 15782, Santiago de Compostela (A Coruña), Spain. E-mail: remigio.paradelo@usc.es; Fax: +34-981594912; Tel: +34-981563100
bDepartamento de Enxeñería Química, E.T.S. de Enxeñeiros Industriais, Universidade de Vigo, Campus Universitario Lagoas Marcosende, 36200 Vigo, Spain
cComplexo Medioambiental de Tratamento de RSU e Asimilables da Mancomunidade de Concellos Serra do Barbanza, Fomento de Construcciones y Contratas, S.A.
First published on 19th November 2010
Abstract
Trace element contamination is one of the main problems linked to the quality of compost, especially when it is produced from urban wastes, which can lead to high levels of some potentially toxic elements such as Cu, Pb or Zn. In this work, the distribution and bioavailability of five elements (Cu, Zn, Pb, Cr and Ni) were studied in five Spanish composts obtained from different feedstocks (municipal solid waste, garden trimmings, sewage sludge and mixed manure). The five composts showed high total concentrations of these elements, which in some cases limited their commercialization due to legal imperatives. First, a physical fractionation of the composts was performed, and the five elements were determined in each size fraction. Their availability was assessed by several methods of extraction (water, CaCl2–DTPA, the PBET extract, the TCLP extract, and sodium pyrophosphate), and their chemical distribution was assessed using the BCR sequential extraction procedure. The results showed that the finer fractions were enriched with the elements studied, and that Cu, Pb and Zn were the most potentially problematic ones, due to both their high total concentrations and availability. The partition into the BCR fractions was different for each element, but the differences between composts were scarce. Pb was evenly distributed among the four fractions defined in the BCR (soluble, oxidizable, reducible and residual); Cu was mainly found in the oxidizable fraction, linked to organic matter, and Zn was mainly associated to the reducible fraction (iron oxides), while Ni and Cr were mainly present almost exclusively in the residual fraction. It was not possible to establish a univocal relation between trace elements availability and their BCR fractionation. Given the differences existing for the availability and distribution of these elements, which not always were related to their total concentrations, we think that legal limits should consider availability, in order to achieve a more realistic assessment of the risks linked to compost use.
Environmental impactThe important role played by composting within the current municipal waste management schemes implies that the production of urban waste composts will increase during the next years. The accurate estimation of trace element availability in composts is becoming more important, as it is of concern not only for agricultural purposes, but also for animal and human health. Therefore, it is necessary to carry out more in depth studies into the risks derived from the use of composts with high concentrations of potential contaminants, and to understand their mobility and speciation and their associated risks. This knowledge must lead to the development and adoption of standardized sound methods that may serve as a basis for laying down new legal limits based on available concentrations. |
Introduction
The presence of high concentrations of some potentially toxic elements (especially Cu, Pb, or Zn) in certain composts raises serious concerns about the adverse environmental impact derived from compost addition to soil. When excessive accumulation of those elements in soil occurs, they may produce adverse effects on the growth and development of plants and may also enter the food chain, thus affecting animal and human health.1 In fact, the total content of elements such as Cu, Pb, Zn, Ni, Cr or Cd is one of the main restrictions to the agricultural use of compost, and in many countries this is the only aspect of compost quality regulated in official thresholds. Nowadays, risk assessment and remediation efforts acknowledge that the total concentrations of potentially toxic elements are not the best indicators of their availability, and that these data are insufficient for risk assessment given that mobility and toxicity depend on their speciation.2,3 The accurate estimation of trace element availability in composts is becoming more important, and several extracting procedures using chemical reagents have been proposed for its assessment.4 As the evaluation of the bioavailability of potentially toxic elements is of concern not only for agricultural purposes, but also for environmental health, more work has to be done to understand the mobility and speciation of these elements present in compost and their associated risks. This knowledge must lead to the development of standardized methods that may serve as a basis for laying down thresholds which take into consideration the available concentrations.5As there is a particular concern about the environmental risks of compost produced from municipal solid waste (MSW) and/or sewage sludge, this work focuses on composts obtained from urban wastes. MSW composts are often contaminated due to the inadequate separation of MSW biodegradable fractions from non-degradable or inert materials.6 In addition to the obvious point sources of contamination, such as batteries, other materials can contribute to the trace element burden of MSW composts, for example paints, electronic, ceramics, plastics,…7 Sewage sludge composts often show higher concentrations of potentially toxic elements (i.e. Cu, Pb, Zn, Cd,…) than other composts,4 which in this case can also be due to the corrosion of canalizations.8 In contrast, green waste composts and manure composts are usually poorer in trace elements,9 although high concentrations of particular elements (for example Zn or Cu) can occur due to metal contamination of plant trimmings or trace element supplements in animal feedings. However, the important role played by composting within the current municipal waste management schemes in many places implies that the production of urban waste composts will increase during the next years. Therefore, it is necessary to carry out more in depth studies into the potential risks derived from the use of composts which may present high concentrations of potential contaminants.
The aim of this study was to assess the relationships between the total concentrations of trace elements, their distribution and bioavailability, and their potential toxicity, using composts produced in Spain, with high concentrations of those elements within the context of the legal limits established for compost commercialization in the Spanish Fertilizers Law. Five elements (Cu, Zn, Pb, Cr and Ni) were selected for their study in the composts, following the general scheme described in Fig. 1. They were first analyzed in the bulk composts and in different particle size fractions. Second, availability was assessed using different extracting agents (the Toxicity Characteristic Leaching Procedure extract and sodium pyrophosphate), and third, their chemical distribution was assessed using the BCR (Bureau Communautaire de Référence) sequential extraction procedure. In order to allow for a complete discussion of the composition of the composts, additional data (availability in water, in CaCl2–DTPA, and in the Physiologically Based Extraction Test extract) previously published9,10 have also been included in the paper.
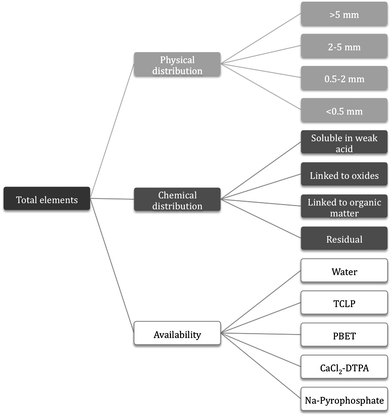 | ||
| Fig. 1 Scheme of the experimental work concerning trace element concentrations. | ||
The main goal of the paper was to show the differences existing in the distribution and availability of trace elements in urban waste compost, as well as the different behaviour of each element with respect to its total concentration. We think that this will demonstrate the need of taking into consideration trace element availability in the legislation. Besides, comparing the results of sequential and single extractions of trace elements in the same composts, which has rarely been done in compost analysis, will provide additional information on the relationships between their chemical forms and their availability.
Experimental
Composts
The composts evaluated were the following: MSWC1 is a compost obtained by anaerobic fermentation of the biodegradable fraction of MSW, separated before collection, followed by an aerobic composting step, to stabilize the incompletely digested residue. MSWC2 is an aerobic MSW compost obtained from the source separated organic fraction of MSW; both composts were provided by industrial composting facilities in Galicia (Spain). MSGW is a commercial compost obtained from source separated MSW mixed with green waste, and MGSS is a compost from municipal garden trimmings mixed with sewage sludge; they both were supplied by an industrial composting facility in Catalunya (Spain). MV is a mixed manure vermicompost supplied by a local producer in Galicia (Spain). All samples were in most cases commercial samples provided by the own producers, with volumes around 50 L. They were composite samples taken by the producers, and they were representative of a compost pile.For the analysis of the general properties of the composts, the Spanish UNE-EN version of the European CEN/TC 223 methods for the characterization of soil amendments and substrates11–15 was followed; these included pH, electrical conductivity (EC), total organic matter (OM) and C, total N, and total trace elements, extracted after wet digestion of the ground sample with aqua regia (HCl and HNO3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio); two replicates of each sample were analyzed for the general characterization. The aqua regia extracts were analyzed for Ni, Cu, Zn, Pb, Cd and Cr using flame atomic absorption spectrometry (Varian SpectraAA 220FS). To confirm the accuracy of the extraction procedure and analysis, the certified reference material CRM143 was also analyzed in parallel to the compost samples. The humic substances were extracted following the Spanish official method for the analysis of organic amendments.16 0.5 g of dry sample was extracted at room temperature for 1 hour with 100 mL of 0.1 M NaOH + 0.1 M Na4P2O7 solution. The extracts were then centrifuged at 4500 rpm for 25 minutes and the procedure repeated twice, mixing all the extracts and adjusting them to 1000 mL. The extractable carbon concentrations (Cext) were determined by the wet dichromate oxidation method. Total carbonates were measured in a Bernard calcimeter according to the method described by Guitián and Carballas.17
1 ratio); two replicates of each sample were analyzed for the general characterization. The aqua regia extracts were analyzed for Ni, Cu, Zn, Pb, Cd and Cr using flame atomic absorption spectrometry (Varian SpectraAA 220FS). To confirm the accuracy of the extraction procedure and analysis, the certified reference material CRM143 was also analyzed in parallel to the compost samples. The humic substances were extracted following the Spanish official method for the analysis of organic amendments.16 0.5 g of dry sample was extracted at room temperature for 1 hour with 100 mL of 0.1 M NaOH + 0.1 M Na4P2O7 solution. The extracts were then centrifuged at 4500 rpm for 25 minutes and the procedure repeated twice, mixing all the extracts and adjusting them to 1000 mL. The extractable carbon concentrations (Cext) were determined by the wet dichromate oxidation method. Total carbonates were measured in a Bernard calcimeter according to the method described by Guitián and Carballas.17
Particle size fractionation
A study into the composition of the composts taking into consideration their different size fractions was performed. Four size fractions were separated for each compost after sieving 400 g of air-dried composts through the following meshes: 0.5, 2, and 5 mm. Each fraction separated was ground in an agate mortar before analysis. Total C and total N were determined by dry combustion using a Leco CNS-2000 (Leco Corp., St Joseph, MI), and the C to N ratio was calculated. Total Fe, Cu, Zn, Pb, Cr and Ni were determined in each fraction after wet digestion with aqua regia, as explained above.Trace element mobility and bioavailability
Several measures of mobility and bioavailability were determined in the bulk composts. In this work we specifically determined the trace elements extractable by the standard Toxicity Characteristic Leaching Procedure (TCLP), a widely spread leaching test, and by sodium pyrophosphate, which solubilizes elements linked to organic matter. These determinations are complimentary to the trace elements extractable in water, CaCl2–DTPA, and the Physiologically Based Extraction Test (PBET) extract, published in two previous papers,9,10 which have also been added to this paper in order to allow for a complete discussion of compost quality.The TCLP was performed in the composts according to the EPA Method 1311.18 The extraction of the soluble elements was performed using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 compost
16 compost![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio after adjusting pH to 4.5 ± 0.1 with acetic acid. After 24 hours of extraction, the volume was adjusted following the equation:
water ratio after adjusting pH to 4.5 ± 0.1 with acetic acid. After 24 hours of extraction, the volume was adjusted following the equation:
| V = 20V − 16W − A |
The sodium pyrophosphate extractable elements were analyzed in the same extract used for determining the humic substances. The concentrations of Cu, Zn, Pn, Ni and Cr were measured directly in the pyrophosphate extract using an ICP-MS spectrophotometer (VARIAN 820-MS).
The water-extractable elements were extracted in distilled water with a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.19 The CaCl2–DTPA extractable elements were extracted with a 0.002 M CaCl2 plus 0.02 M DTPA (diethylene triamine pentaacetic acid) solution at pH 2.6 with a solid
5.19 The CaCl2–DTPA extractable elements were extracted with a 0.002 M CaCl2 plus 0.02 M DTPA (diethylene triamine pentaacetic acid) solution at pH 2.6 with a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.20 The trace elements available to mammals were obtained after performing the Physiologically Based Extraction Test (PBET) following Ruby et al.21 More details on these determinations are available in the two papers mentioned.9,10
5.20 The trace elements available to mammals were obtained after performing the Physiologically Based Extraction Test (PBET) following Ruby et al.21 More details on these determinations are available in the two papers mentioned.9,10
In all determinations, three replicates were performed for each sample and a blank was run in parallel to the analysis.
BCR sequential extraction
The chemical fractionation of the trace elements in the composts was conducted using the BCR sequential extraction procedure as described by Rauret et al.22 This method consists of three steps and a residual extraction. Two independent replications were performed for each sample.The Cu, Pb, Zn, Cr and Ni concentrations in all the BCR extracts were determined using an ICP-MS spectrophotometer (VARIAN 820-MS). Blanks were run in parallel to all the determinations. To confirm the accuracy of the extraction procedure and analysis, the certified reference material CRM701 was also analyzed in parallel to the compost samples. The recovery of the elements in the different fractions was between 83 and 109% of the certified values, and the recovery for the total concentrations was between 90 and 104%.
Phytotoxicity
Finally, a biological test with compost extracts was conducted for evaluating phytotoxicity. It is a widely used germination–elongation test in which a germination index (GI) measuring both seed germination and root elongation is determined following Zucconi et al.24 Aqueous extracts of fresh samples (sample/water ratio: 1/10 w/v) were obtained after two hour shaking, and filtered. The test was run with three species commonly used for compost phytotoxicity determination: Hordeum vulgare L. (spring barley), Lolium multiflorum Lam. (Italian ryegrass) and Lepidium sativum L. (garden cress). A previous soaking step was introduced in the test, as suggested by Wierzbicka and Obidzinska:25 for this, seeds of each species were submerged in 50 mL of the tested extract (or distilled water for the control) for 24 h. Following, twenty seeds of each species were placed on a filter paper in 9 cm Petri dishes (triplicates for each of the 3 test plants) and received a 3 mL aliquot of the compost extract, whereas the controls received the same quantity of distilled water. Petri dishes were sealed to prevent evaporation and incubated in the dark at 25 °C for 120 h. The presence of a 1 mm primary root was used as the operational definition of germination. The number of germinated seeds and root length were measured, in order to calculate the germination index (GI) as follows:where G and L are the germination and radicle growth of the seeds germinated in the tested solution, respectively, and GC and LC the germination and radicle growth of the seeds in the control (distilled water), respectively. From now on, the ratios 100 × (G/GC) and 100 × (L/LC) will be referred to as G (germination) and RG (root growth), respectively, for simplicity. According to Zucconi et al.,24 GI values below 50% are considered indicative of a high toxicity, values between 50% and 80% represent a slight toxicity and when the GI is higher than 80%, no phytotoxicity is considered.
Results and discussion
Properties of the composts
Table 1 shows the general properties of the composts evaluated. Their pH goes from neutral (MGSS) to alkaline (MSGW). The salinity of the MSW composts was the highest, and exceeded the value of 1.5 dS m−1 which is usually regarded as inadequate for the agricultural use of compost.26 All of the composts presented similar concentrations of OM and total N. MSGW and MGSS showed the highest concentration of humic substances (Cext). Additional information on the maturity and stability of the composts can be found in Paradelo et al.27| MSWC1 | MSWC2 | MSGW | MGSS | MV | |
|---|---|---|---|---|---|
| pH | 8.4 | 8.2 | 9.2 | 7.3 | 7.9 |
| EC/dS m−1 | 2.3 | 2.4 | 1.2 | 1.4 | 0.7 |
| OM/g kg−1 | 490 | 397 | 429 | 515 | 376 |
| Total C/g kg−1 | 280 | 230 | 248 | 298 | 217 |
| C ext/g kg−1 | 64 | 56 | 77 | 86 | 43 |
| Total N/g kg−1 | 17 | 15 | 17 | 18 | 10 |
| C/N | 17 | 15 | 14 | 15 | 21 |
| Total carbonates (%) | 16 | 17 | 13 | 16 | 13 |
| Total Cu | 325 | 829 | 52 | 688 | 144 |
| Total Zn | 608 | 1149 | 200 | 896 | 689 |
| Total Cd | 3.5 | 3.1 | 2.1 | 2.7 | 2.0 |
| Total Pb | 188 | 223 | 62 | 180 | 33 |
| Total Cr | 80 | 77 | 17 | 68 | 23 |
| Total Ni | 57 | 75 | 25 | 71 | 27 |
| Classification | Not allowed | Not allowed | C | Not allowed | C |
As for the total trace element concentrations in the composts, Zn and Cu were the most abundant in all the composts, followed by Pb, Cr and Ni, and the last, Cd. The feedstock used for compost production has an important role on the final compost contents. The composts obtained from urban wastes had the highest contents, and they even surpassed the levels allowed in the Spanish regulation for the lowest quality organic amendments.28 According to this law, MSWC1 exceeded the limit for Cd, MSWC2 for Cu, Cd, Pb and Zn, and MGSS for Cu, and they could not be used as organic amendments. Even the composts MV and MSGW, which presented low concentrations of trace elements, would be categorized as class C composts, and therefore could not be used in agricultural soils at rates higher than 5 t ha−1.
The particle size distribution of the composts MSWC1, MSGW and MGSS was very similar, showing a predominance of the fractions <2 mm (Fig. 2). In general, the finer fractions showed lower C/N ratios (Table 2); this can be explained as a normal consequence of compost maturation, which is usually accompanied by a reduction of particle size as a result of decomposition,29 and therefore tends to concentrate the most stable organic matter in the fine fractions. As an exception, the compost MV presented lower C concentrations in the coarser fractions, what is explained by the predominance of granitic gravels and other soil constituents in those fractions in this particular compost.
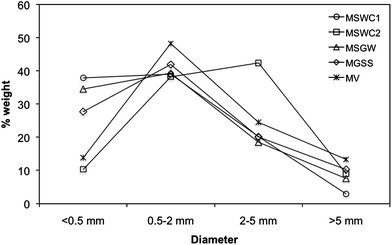 | ||
| Fig. 2 Particle size distribution of the composts. | ||
| MSWC2 | >5 mm | 2–5 mm | 0.5–2 mm | <0.5 mm |
|---|---|---|---|---|
| C (%) | 37.8 | 26.1 | 17.3 | 16.7 |
| N (%) | 1.6 | 1.9 | 1.4 | 1.7 |
| C/N | 24 | 14 | 12 | 10 |
| MSGW | >5 mm | 2–5 mm | 0.5–2 mm | <0.5 mm |
|---|---|---|---|---|
| C (%) | 28.9 | 25.8 | 23.0 | 20.2 |
| N (%) | 1.5 | 1.7 | 1.9 | 2.1 |
| C/N | 19 | 15 | 12 | 10 |
| MGSS | >5 mm | 2–5 mm | 0.5–2 mm | <0.5 mm |
|---|---|---|---|---|
| C (%) | 28.2 | 31.1 | 29.2 | 21.7 |
| N (%) | 1.8 | 2.3 | 2.7 | 2.4 |
| C/N | 16 | 14 | 11 | 9 |
| MV | >5 mm | 2–5 mm | 0.5–2 mm | <0.5 mm |
|---|---|---|---|---|
| C (%) | 2.8 | 9.4 | 11.4 | 11.4 |
| N (%) | 0.4 | 1.0 | 1.1 | 1.2 |
| C/N | 8 | 10 | 10 | 9 |
Trace element particle size fractionation
As shown in Table 3, trace elements were unevenly distributed among the different particle size fractions of the composts. The highest concentrations of Fe appeared in the finer fractions (<2 mm) of MSWC1, MSWC2 and MGSS, whereas MV and MSGW had the highest concentrations in the fraction >5 mm. As for Cu, its concentrations increased in the finer fractions in the composts MSWC1, MSWC2, MGSS and MV, while remaining fairly constant in the compost MSGW. All the composts showed a consistent trend of increasing Zn concentration with decreasing particle size. The concentrations of Pb showed less clear trends than Zn, but in general the fractions under 2 mm were richer than the fractions over 2 mm, with the exception of the compost MV, for which no differences existed. The behaviour of Cr was very similar to that of Fe, with the highest concentrations in the finer fractions in the composts MSWC1, MSWC2 and MGSS, whereas MSGW had the highest concentrations in the fraction >5 mm, and no differences existed for the compost MV. Finally, Ni was overall evenly distributed in the different size fractions for all the composts.| MSWC1 | >5 mma | 2–5 mm | 0.5–2 mm | <0.5 mm |
|---|---|---|---|---|
| a The amount of this fraction in MSWC1 was not sufficient for the determinations. | ||||
| Fe | — | 11.7 ± 0.4 | 18.2 ± 0.1 | 22.2 ± 0.8 |
| Cu | — | 657 ± 10 | 683 ± 9 | 758 ± 34 |
| Zn | — | 731 ± 6 | 907 ± 18 | 969 ± 27 |
| Pb | — | 377 ± 19 | 489 ± 0.1 | 588 ± 2 |
| Cr | — | 31 ± 6 | 46 ± 2 | 56 ± 3 |
| Ni | — | 56 ± 6 | 67 ± 1 | 76 ± 5 |
Several researchers4,30–33 have observed that trace elements are not equally distributed among all physical fractions in compost, and that they are in many cases mainly associated with finer particle size classes, in particular in clusters under 1 mm.4,34 We hypothesized in an earlier paper,33 using magnetic methods, that fine metallic particles might be significant contributors to the total concentrations of these elements in the compost, which is supported by the data presented here. Also, since the finer fractions are also the ones with the highest degree of stability,35 that is, those which have suffered the highest loss of carbon, it is likely that these elements are relatively concentrated in those fractions. As trace elements do not degrade during the composting process, several authors observed that their concentrations are progressively increased during composting precisely as a result of carbon loss.7,36–38 Also, it has been reported that trace elements are transformed into less mobile fractions throughout the process,3,36,39–41 so a reduction of their initial availability is expected during composting. Therefore, the process of compost maturation would lead to a progressive increment of the fine compost fractions, which concentrate increasing amounts of trace elements, in forms expected to be of low availability.
Trace element mobility and bioavailability
Table 4 shows the trace element concentrations of the composts, measured in different extracting agents currently used for estimating different pools of available elements. Water is expected to extract those metals that will be immediately transferred from compost to the soil solution, and the TCLP simulates the leaching of trace elements in a slightly acidic medium. Chelating agents such as DTPA are widely used to assess the short-term plant availability of trace elements.42–45 The PBET is expected to assess the pool available to mammals,21 whereas sodium pyrophosphate extracts the pool complexed with organic matter. Overall, water and the diluted acetic acid used in the TCLP were the mildest extracting agents, with extraction percentages under 5%, and CaCl2–DTPA and sodium pyrophosphate the strongest, reaching in some cases extraction percentages over 50%, while the PBET always rendered intermediate results, close for Zn to those of CaCl2–DTPA.| MSWC1 | Watera | TCLP | PBETb | CaCl2–DTPAa | Na-pyrophosphate |
|---|---|---|---|---|---|
| a Data from ref. 9. b Data from ref. 10. c Not determined. | |||||
| Cu | 15 ± 0.2 (4.6) | 2.7 ± 0.01 (0.8) | 28 ± 2 (8.6) | 82 ± 8 (25) | 250 ± 14 (77) |
| Zn | 10 ± 0.4 (1.6) | 5.3 ± 0.9 (0.9) | 273 ± 32 (45) | 231 ± 3 (38) | 566 ± 3 (93) |
| Pb | 5.7 ± 1.1 (3.0) | 0.2 ± 0.05 (0.1) | 29 ± 4 (15) | 127 ± 2 (68) | 78 ± 9 (41) |
| Cr | 0.8 ± 0.03 (1.0) | <0.08 | 2.0 ± 0.2 (2.5) | 1.7 ± 0.0 (2.1) | <5 |
| Ni | <0.5 | 0.4 ± 0.0 (0.7) | 5.1 ± 0.8 (8.9) | 5.8 ± 0.0 (10) | 8 ± 5 (13) |
| MSWC2 | Water | TCLP | PBET | CaCl2–DTPA | Na-pyrophosphate |
|---|---|---|---|---|---|
| Cu | 13 ± 0.3 (1.6) | 4.8 ± 0.3 (0.6) | 27 ± 7 (3.3) | 153 ± 4 (18) | 325 ± 12 (39) |
| Zn | 9.6 ± 0.2 (0.8) | 9.3 ± 1.5 (0.8) | 216 ± 49 (19) | 267 ± 3 (23) | 604 ± 1 (53) |
| Pb | 8.9 ± 1.1 (4.0) | 0.4 ± 0.2 (0.2) | 10 ± 7 (4.4) | 92 ± 9 (41) | <15 |
| Cr | 0.4 ± 0.03 (0.5) | 0.3 ± 0.01 (0.4) | <0.5 | 0.7 ± 0.0 (0.9) | <5 |
| Ni | <0.5 | 0.7 ± 0.2 (0.9) | 3.0 ± 0.7 (4.0) | 5.1 ± 0.0 (6.8) | 9 ± 6 (12) |
| MSGW | Water | TCLP | PBET | CaCl2–DTPA | Na-pyrophosphate |
|---|---|---|---|---|---|
| Cu | 0.7 ± 0.1 (1.3) | 0.4 ± 0.1 (0.8) | 0.3 ± 0.2 (0.6) | 3.3 ± 0.0 (6.3) | 52 ± 1 (100) |
| Zn | 1.4 ± 0.1 (0.7) | 1.2 ± 0.2 (0.6) | 35 ± 8 (18) | 41 ± 0.4 (21) | 128 ± 0.1 (64) |
| Pb | 3.8 ± 0.1 (6.1) | 0.4 ± 0.2 (0.6) | 2 ± 3 (3.4) | 34 ± 1 (55) | <15 |
| Cr | <0.3 | <0.08 | <0.5 | 0.4 ± 0.0 (2.4) | <5 |
| Ni | <0.5 | <0.08 | <0.5 | 0.7 ± 0.1 (2.8) | <5 |
| MGSS | Water | TCLP | PBET | CaCl2–DTPA | Na-pyrophosphate |
|---|---|---|---|---|---|
| Cu | 0.2 ± 0.01 (0.03) | 0.5 ± 0.02 (0.1) | 1.2 ± 0.4 (0.2) | 6.1 ± 0.6 (0.9) | 69 ± 2 (10) |
| Zn | 0.5 ± 0.03 (0.1) | 1.1 ± 0.03 (0.1) | 40 ± 5 (4.5) | 50 ± 1 (5.6) | 191 ± 3 (21) |
| Pb | 3.9 ± 0.5 (2.2) | 0.8 ± 0.3 (0.4) | <1.5 | 34 ± 2 (19) | <15 |
| Cr | <0.3 | <0.08 | <0.5 | 0.5 ± 0.0 (0.7) | <5 |
| Ni | <0.5 | 0.2 ± 0.1 (0.3) | <0.5 | 0.8 ± 0.0 (1.1) | <5 |
| MV | Water | TCLP | PBETc | CaCl2–DTPA | Na-pyrophosphate |
|---|---|---|---|---|---|
| Cu | 0.4 ± 0.03 (0.3) | 1.0 ± 0.1 (1.2) | — | 32 ± 0.3 (22) | 102 ± 4 (71) |
| Zn | 1.1 ± 0.03 (0.2) | 1.7 ± 0.02 (0.2) | — | 228 ± 5 (33) | 550 ± 4 (80) |
| Pb | 3.3 ± 0.2 (10) | 0.3 ± 0.04 (0.9) | — | 17 ± 0.3 (52) | <15 |
| Cr | <0.3 | 0.2 ± 0.05 (0.9) | — | 0.8 ± 0.0 (3.5) | <5 |
| Ni | <0.5 | 0.2 ± 0.05 (0.7) | — | 1.7 ± 0.0 (6.3) | <5 |
The TCLP was designed to determine the mobility of contaminants present in solid and multiphasic wastes.18 This procedure extracted concentrations similar to those extracted by water (Table 4), which represented less than 2% of the total concentrations, thus indicating a low level of potential environmental risk. The extractability sequence was as follows: Zn > Cu > Pb > Ni, while Cr was not extractable. Chiang et al.45 observed a similar extractability sequence for sewage sludge compost: Zn > Ni > Cu > Pb = Cr, although higher soluble concentrations were obtained in comparison with those found in our work. Ni showed a lower availability in the composts studied here than in the literature, and the same happened for water-extractable Ni.36,45
Sodium pyrophosphate is expected to selectively extract those fractions of elements complexed with organic matter,46 although exchangeable fractions are also expected to be mobilized. This fraction should represent the maximum amount of element which would be released to soil upon the complete mineralization of compost organic matter.47 In the composts studied here, the elements extracted by sodium pyrophosphate were much higher than those extracted with CaCl2–DTPA (Table 4). Cu and Zn were extracted to a great extent in all composts, reaching in some cases the total concentration of the element. Ni was only extracted in MSWC1 and MSWC2, and Pb in MSWC1, whereas Cr was not present in the pyrophosphate extract of any compost. Taking into account the importance of this fraction for Cu and Zn, it follows that an important part of them is linked to organic matter. The high extractability of Zn in pyrophosphate has already been noted by Genevini et al.47 and He et al.48 However, our results for Pb, Ni and Cr disagree with those reported for MSW composts by Genevini et al.,47 who found high extraction percentages for these elements, with maxima extraction percentages of 96% for Pb, 81% for Ni, and 71% for Cr. In the composts studied here, Ni and Cr were the metals with the lowest extraction percentages in this agent, thus indicating that they are not linked to the organic fraction of the MSW, but rather to other components such as metals. This corroborates our conclusions from a previous work, showing that these elements are mainly linked to magnetic metallic particles.33
The relative extractability sequences showed that, in all cases, Pb, Cu and Zn were the most available elements, although some differences existed in the sequence of these three elements between the extractants: Pb > Cu > Zn in water, Zn > Cu > Pb in the TCLP and the pyrophosphate extracts, Pb > Zn > Cu in CaCl2–DTPA, and Zn > Pb > Cu in the PBET. The low availability found for Ni and Cr in all the extracting agents can be explained on the basis of their association to Fe in alloys, as they have been found to be linked to magnetic particles in compost.33
BCR sequential extraction
Table 5 and Fig. 3 show the results of the BCR sequential extraction procedure. The results of the previous extractions indicated differences in the availability of each element, which may be originated in their partition into different chemical fractions in the compost.| Step 1 | Step 2 | Step 3 | Residual | |
|---|---|---|---|---|
| MSWC1 | ||||
| Cu | 11 ± 0.4 | 31 ± 0.7 | 162 ± 3 | 57 ± 10 |
| Zn | 125 ± 2 | 357 ± 19 | 27 ± 2 | 58 ± 12 |
| Pb | 12 ± 2 | 154 ± 13 | 62 ± 2 | 54 ± 33 |
| Cr | <2 | 5.7 ± 0.2 | 15 ± 0.1 | 52 ± 4 |
| Ni | 7.1 ± 0.3 | 15 ± 0.1 | 7 ± 2 | 37 ± 13 |
| MSWC2 | ||||
| Cu | 6.7 ± 0.5 | 26 ± 0.4 | 353 ± 24 | 53 ± 2 |
| Zn | 121 ± 2 | 565 ± 5 | 43 ± 5 | 47 ± 5 |
| Pb | 11 ± 0.2 | 86 ± 1 | 93 ± 13 | 75 ± 3 |
| Cr | <2 | 2.8 ± 0.02 | 15 ± 2 | 83 ± 52 |
| Ni | 7.9 ± 0.3 | 18 ± 0.1 | 14 ± 2 | 35 ± 24 |
| MSGW | ||||
| Cu | <1 | <1 | 21 ± 2 | 16 ± 0.6 |
| Zn | <0.4 | 108 ± 1 | 21 ± 0.1 | 41 ± 3 |
| Pb | 11 ± 1 | 17 ± 1 | 17 ± 0.6 | 19 ± 1 |
| Cr | <2 | <2 | <2 | 14 ± 0.04 |
| Ni | 5.1 ± 0.4 | 0.7 ± 0.3 | 1.1 ± 0.2 | 8 ± 1 |
| MGSS | ||||
| Cu | 3.8 ± 2 | 4.6 ± 6 | 103 ± 20 | 15 ± 6 |
| Zn | 5.2 ± 3 | 151 ± 51 | 27 ± 4 | 26 ± 0.7 |
| Pb | 10 ± 2 | 17 ± 2 | 22 ± 0.5 | 14 ± 0.03 |
| Cr | <2 | 2.4 ± 0.1 | 2.7 ± 0.7 | 13 ± 2 |
| Ni | 4.4 ± 0.2 | 1.8 ± 0.2 | 1.2 ± 0.8 | 4.7 ± 0.5 |
| MV | ||||
| Cu | 2.2 ± 0.3 | 15 ± 0.9 | 31 ± 5 | 24 ± 0.6 |
| Zn | 137 ± 3 | 305 ± 15 | 17 ± 11 | 35 ± 3 |
| Pb | 6.4 ± 0.6 | 1.0 ± 0.8 | 10 ± 2 | 16 ± 1 |
| Cr | <2 | 3.9 ± 0.1 | <2 | 16 ± 2 |
| Ni | 4.2 ± 0.7 | 2.2 ± 0.6 | 0.4 ± 0.3 | 9 ± 2 |
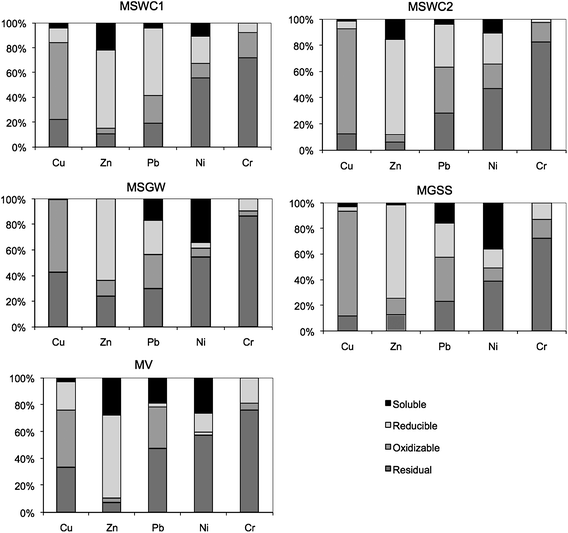 | ||
| Fig. 3 Results of the BCR sequential extraction procedure, in percentage. | ||
In the five composts studied, Cu was mainly found in the oxidizable BCR fraction (step 3), followed by the residual fraction. It is well known that this element has a great affinity for organic matter,49 and several authors have found similar results in composts from different sources.2,3,38,50
Zinc was mainly associated to oxides (step 2) in all the composts together, with important acid-soluble pools (step 1) in MSWC1, MSWC2 and MV. Tisdell and Breslin2 also found that Zn was mainly linked to oxides in MSW compost, whereas Greenway and Song3 and Farrell and Jones38 found that this element is evenly distributed between the four fractions of the BCR scheme.
Overall, Pb was evenly distributed among the four fractions in the five composts, although MSWC1 had an important concentration in the reducible fraction (step 2). It is noteworthy that all the composts had significant Pb concentrations in the acid-soluble fraction (step 1). Tisdell and Breslin2 found this element evenly distributed between oxides, organic matter, and residual fractions, whereas Farrell and Jones39 found Pb mainly in the reducible and oxidizable fractions. Greenway and Song3 found that most Pb exists in forms combined with organic matter or in the residual fraction, in agreement with Wong and Selvam.50 The compost MV had the most similar behaviour to that reported by these last authors.
Chromium was present almost exclusively in the residual fraction in all the composts. The reducible and oxidizable fractions presented similar overall Cr concentrations, whereas this element was not detected in the acid-soluble fractions in any compost. These results agree with the low availability observed in the analyses above. Tisdell and Breslin2 and Greenway and Song3 observed that this element was mainly bound to the residual and organic fractions in compost.
Nickel mainly appeared in the residual fraction of the composts, although most of them presented also a high acid-soluble fraction for this element. He et al.48 pointed out that the major fraction of Ni in MSW compost resides in the residual fraction, probably as metallic or alloy forms, silicate minerals, glass, and plastics. In agreement with this, Farrell and Jones38 found Ni mainly in the residual fraction of MSW and green waste composts. Tisdell and Breslin2 found this element evenly distributed between the oxide-bound, organic matter-bound and residual fractions, whereas Wong and Selvam50 found Ni mainly in the oxide-bound and residual fractions in composted sewage sludge. Notwithstanding, other authors consider Ni as a potentially mobile and water-soluble metal, as being weakly linked to the compost matrix compared to other metals,4 and for example Greenway and Song3 found 20–40% of Ni in the acid-soluble fraction, although a wide variation existed among different feedstocks. This also agrees with the size of the acid-soluble fraction found in the composts studied here.
The comparison of the operationally defined fractions of the BCR procedure with the availability assessed by means of the single extractants shows that it is not easy to make an availability assessment based on this procedure. The correlations found between the fractions of the BCR scheme and most of the single extractions (Table 6) do not help clarifying this question. The first and second fractions of the BCR are highly correlated with the results of almost all of the single extractants, whereas unexpectedly, the third fraction correlates only with the results of the water extraction, although it aims at the same pools than the pyrophosphate and the CaCl2–DTPA extractions. However, the quantities extracted with the different methods are not coincident between them, although high correlations had been found, so making comparisons on the basis of the correlations only is not possible.
Sequential extractions were designed for the selective extraction of trace elements from operationally defined solid fractions,51–53 and although these procedures are not fully specific in extracting the element bound to a given solid fraction, they may provide comparative information on their mobility in changing environmental conditions, such as pH or redox potential. Although the use of sequential extraction procedures in soil analysis as a complement to single extractions has increased,23 the BCR procedure has been rarely applied to the analysis of compost3 or MSW.54 Composts are extremely heterogeneous materials with a high organic matter content, and these two features differentiate them from soils or sediments, for which the BCR sequential extraction procedure was developed. Therefore, the interpretation of the results may be different in this case, as the significance of the operationally defined fractions may vary from the original ones. For example, the acid-soluble fraction of the BCR scheme (step 1) can have an ambiguous significance in terms of availability, as it is not exactly equivalent to an exchangeable or water-soluble fraction. This step is expected to dissolve carbonates as well as water-soluble and exchangeable elements. The elements extracted in this fraction are considered readily and potentially bioavailable, and the results could be roughly compared to those of the TCLP extraction. Nevertheless, the amounts extracted in the composts studied were higher in this BCR fraction than those found in water or in the TCLP extracts, specially for Zn, Pb, and Ni, but lower than those in the CaCl2–DTPA or PBET extracts, what means that this fraction has an intermediate degree of availability. This BCR fraction usually contains a small percentage of the total concentration of elements,46 and it is unlikely that the relatively high Zn and Ni concentrations found in this fraction correspond uniquely to water-soluble or exchangeable forms. Instead, the existence of metals occluded or adsorbed onto carbonates cannot be neglected, as it is usual that MSW present important amounts of carbonates,55 and indeed all the composts studied presented significant concentrations of carbonates (Table 1). Tisdell and Breslin,2 for example, found important percentages of carbonate-bound heavy metals in MSW composts (especially for Zn and Pb). This fact can explain why Ni was found to have a low availability and at the same time, an important acid-soluble fraction. Moreover, elements linked to carbonates are expected to show different availability as a function of soil conditions: for example, the addition of an alkaline compost to an acid soil will immediately produce the solubilisation of carbonates and thus the acid-soluble elements will be available to plants. In turn, if the compost is added to a neutral soil, those elements will remain unavailable to plants, as they will not be solubilised at the soil pH. Having this in mind, we think that this fraction is expected to give a better prediction of availability in compost-amended acid soils than water or other extractants (such as diluted salts) used for assessing availability in the short-term.
The step 2 of the BCR scheme extracts elements linked to Fe and Mn oxides. The reduction of Fe(III) and Mn(IV) under anoxic conditions and the subsequent dissolution can lead to the release of the elements in this fraction,46 although they can be considered rather immobile under common soil redox conditions. Consequently Pueyo et al.23 indicated that the results of this fractionation step must not be used for the prediction of plant availability. Oxides are expected to be less important in composts or sewage sludge than in soils or sediments, so the size of these fractions in compost should be expected to be small. Among the five elements considered, Zn was the only clearly linked to this fraction. However, the high concentrations found in this fraction might be overestimated at the expense of the oxidizable fraction, as hydroxylamine hydrochloride in nitric acid can release substantial amounts of trace elements bound to organic matter.56
The step 3 of the BCR scheme is expected to extract those elements linked to organic matter. Pollutants associated with oxidizable fractions are assumed to remain in the soil for long periods, but may be mobilized by decomposition processes or under the action of complexing agents such as organic acids. Degradation of organic matter under oxidizing conditions is expected to release these elements to the environment. Nevertheless, in the short term, the elements extracted in this fraction are not considered very mobile or available, due to their association to stable humic substances.46 Moreover, it has been observed that OM mineralization in stable composts is very low during the first months or even years after application to soil.57–60 In the composts studied here, Cu was the element most clearly linked to this fraction. It is obvious, by comparing the size of the BCR oxidizable fraction with the amounts extracted by CaCl2–DTPA, that the last does not extract the whole organic matter-complexed elements. There were also differences in the extractive strength of pyrophosphate and H2O2, which is employed in this BCR fraction. Sodium pyrophosphate always mobilized higher amounts of Zn than H2O2 did, what can be attributed to the additional extraction of water-soluble and exchangeable Zn in the first case. The amounts of Cu extracted with pyrophosphate were equal or higher to those of the BCR oxidizable fraction. However, for Pb the relative concentrations in the two extracts were the inverse, except for the compost MSWC1, what could be attributed to reduced mobility of Pb at the alkaline pH of the pyrophosphate extraction, as this element is very sensitive to pH changes.
Finally, the residual fraction of the BCR procedure comprises elements occluded in crystalline lattices, which are virtually non available. In the case of compost, it has to be kept in mind that this fraction can include not only phyllosilicates and other minerals, but also important amounts of metallic particles and other inert materials present in MSW, such as plastics, glass,… which are not dissolved by the precedent reagents of the procedure. This was the main fraction for Cr and Ni in the composts, with lesser quantities for Cu, Pb and Zn.
The difficulties found to link the operationally defined fractions of the BCR scheme either to trace element availability in compost or to distinct element pools (such as water-soluble or exchangeable) makes it essential to make further studies at a field scale and plant assays in order to adequately assess the utility of this scheme to study compost heavy metals. In particular, the disagreements observed between the pyrophosphate single extraction and the oxidizable fraction of the BCR scheme suggest that the last is not adequately focused on the organic fraction of the compost, and it is suspected that some elements associated with organic matter can be mobilized in the first steps of the fractionation, specially in the second one,56 thus underestimating in some cases the pool of organically bound elements.
Phytotoxicity
As it is indicated in Table 7, strong phytotoxicity (GI < 50) was not observed for any of the composts, whereas depending on the species employed, moderate phytotoxicity was found for the composts MSWC1, MSWC2 and MGSS. It can be seen that RG had values similar to GI, while G was less variable, thus confirming the opinion of previous works61,62 that root elongation could be used alone as an indicator of phytotoxicity, due to its higher sensitivity to phytotoxic factors when compared to the germination percentage. The moderate phytotoxicity found in some composts, however, cannot be attributed to their trace element concentrations. In a previous work, we have found that the water-soluble concentrations of Cu, Pb and Zn which could produce a phytotoxic response in the conditions of the germination test assayed here would be around 50 mg kg−1 for Cu and Pb, and around 250 mg kg−1 for Zn.63 As these values are considerably higher than those shown in Table 4 for the composts, it seems that these elements are not the main cause of phytotoxicity, but the high salinity found in MSW composts.| Barley | Cress | Ryegrass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G | RG | GI | G | RG | GI | G | RG | GI | |
| MSWC1 | 94 ± 6 | 78 ± 3 | 74 ± 1 | 95 ± 8 | 131 ± 8 | 124 ± 15 | 77 ± 3 | 111 ± 26 | 86 ± 21 |
| MSWC2 | 94 ± 6 | 92 ± 2 | 87 ± 5 | 95 ± 13 | 74 ± 5 | 71 ± 5 | 83 ± 14 | 122 ± 24 | 100 ± 20 |
| MSGW | 106 ± 6 | 92 ± 10 | 97 ± 8 | 98 ± 5 | 115 ± 20 | 114 ± 26 | 89 ± 9 | 117 ± 25 | 104 ± 29 |
| MGSS | 94 ± 6 | 53 ± 8 | 51 ± 9 | 100 ± 6 | 104 ± 11 | 104 ± 8 | 74 ± 6 | 107 ± 17 | 78 ± 6 |
| MV | 93 ± 3 | 93 ± 12 | 86 ± 8 | 98 ± 5 | 132 ± 4 | 129 ± 11 | 79 ± 17 | 113 ± 14 | 89 ± 18 |
Conclusion
The distribution and bioavailability of Cu, Pb, Zn, Cr and Ni in five composts produced in the Spanish industry were studied by different methods. It has to be noted that the composts analyzed were selected because they present high concentrations of those elements, often problematic in composts, and in some cases surpassing the levels allowed for commercialization in Spanish regulation. However, it is a subject of discussion if the total concentrations of trace elements can be the only matter to be kept in mind when assessing the environmental and health risks associated to composts. The distribution, mobility and bioavailability of these elements can be different for equal total concentrations, given the different nature and properties of the materials employed for the production of compost. In addition, trace elements are unevenly distributed between both physical and chemical fractions, what will surely influence their bioavailability.In the composts studied here, the fine size particles were overall enriched in trace elements with respect to the coarse fractions, except for Ni, whose concentrations did not vary with particle size. Also, the distribution in different particle size of the elements was different for each compost.
The chemical fractionation was also different for each trace element. Pb was evenly distributed among the soluble, oxidizable, reducible and residual fractions of the BCR scheme; Cu was mainly found in the oxidizable fraction, linked to organic matter, and Zn was mainly associated to iron oxides (reducible fraction), while Ni and Cr were present almost exclusively in the residual fraction. The differences in the fractionation of each between compost were small compared to those existing between elements.
Bioavailability, assessed using different single extracting agents, was also different for each element. Overall, Cu and Zn were the most problematic elements in terms of potential risk, as they both presented the highest percentages of extractability and the highest total concentrations in each extractant. However, it was not possible to link the operationally defined fractions of the BCR scheme to trace element availability as shown by these extracting agents.
Finally, it was concluded that the concentrations of trace elements are probably too low to be the cause of compost phytotoxicity. The difficulties found for the attribution of phytotoxicity to these elements, even when they surpass the total concentrations allowed for compost commercialization, reinforce our thesis that the normative regulating compost marketing should contemplate some measure of availability in addition to the total concentrations.
Our results suggest that the legal limits for potentially toxic trace elements in compost, which at this moment are mostly regulated on the basis of their total contents, should also be assessed on the basis of their availability, at least for the elements which are usually present in the highest mobile forms, such as Cu, Pb and Zn.
Acknowledgements
The authors acknowledge the analytical assistance of Ms Montserrat Recarey. This investigation was supported by the Xunta de Galicia Regional Government (Project PGIDIT06TAM014E).References
- A. Kabata-Pendias and H. Pendias, Trace Elements in Soils and Plants, CRC Press, Boca Raton, 1984 Search PubMed.
- S. E. Tisdell and V. T. Breslin, J. Environ. Qual., 1995, 24, 827–833 CrossRef CAS.
- G. M. Greenway and Q. J. Song, J. Environ. Monit., 2002, 4, 300–305 RSC.
- S. R. Smith, Environ. Int., 2009, 35, 142–156 CrossRef CAS.
- M. T. Barral and R. Paradelo, Waste Manage. Res. DOI:10.1016/j.wasman.2010.10.019.
- R. T. Haug, The Practical Book of Compost Engineering, Lewis Publishers, Boca Raton, 1993 Search PubMed.
- M. Farrell and D. L. Jones, Bioresour. Technol., 2009, 100, 4301–4310 CrossRef CAS.
- R. L. Chaney, J. A. Ryan, U. Kukier, S. L. Brown, G. Siebielec, M. Malik and J. S. Angle, in Utilización de Compost en Los Sistemas de Cultivo Hortícolas, ed. P. J. Stofella and B. A. Kahn, Mundi-Prensa, Madrid, Spain, 2004, pp. 323–358 Search PubMed.
- M. T. Barral, A. B. Moldes, Y. Cendón and F. Díaz-Fierros, Waste Manage. Res., 2007, 25, 99–108 Search PubMed.
- R. Paradelo Núñez, R. Devesa Rey, A. B. Moldes Menduíña and M. T. Barral Silva, J. Trace Elem. Med. Biol., 2007, 21(S1), 83–85 CrossRef.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Determinación del pH: Norma Española UNE-EN 13037, AENOR, Madrid, 2001 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Determinación de la Conductividad Eléctrica: Norma Española UNE-EN 13038, AENOR, Madrid, 2001 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Determinación del Contenido en Materia Orgánica y de las Cenizas: Norma Española UNE-EN 13039, AENOR, Madrid, 2001 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Extracción de Elementos Solubles en Agua Regia: Norma Española UNE-EN 13650, AENOR, Madrid, 2002 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Determinación de Nitrógeno: Norma Española UNE-EN 13654-1, 13654-2, AENOR, Madrid, 2002 Search PubMed.
- Ministry of Agriculture, Métodos Oficiales de Análisis. Tomo III: Plantas, Productos Orgánicos Fertilizantes, Suelos, Agua, Productos Fitosanitarios y Fertilizantes Inorgánicos, Publicaciones del MAPA, Madrid, 1991 Search PubMed.
- F. Guitián and T. Carballas, Técnicas de Análisis de Suelos, Pico Sacro, Santiago, Spain, 1976 Search PubMed.
- USEPA, Method 1311: Toxicity Characteristic Leaching Procedure (TCLP), US Environmental Protection Agency, Washington, DC, 1992 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Extracción de Nutrientes y Elementos Solubles en Agua: Norma Española UNE-EN 13652, AENOR, Madrid, 2002 Search PubMed.
- AENOR (Asociación Española de Normalización y Certificación), Mejoradores del Suelo y Sustratos de Cultivo: Extracción de Nutrientes Solubles en Cloruro Cálcico-DTPA (CAT): Norma Española UNE-EN 13651, AENOR, Madrid, 2002 Search PubMed.
- M. V. Ruby, A. Davis, R. Schoof, S. Eberle and C. Sellstone, Environ. Sci. Technol., 1996, 30, 422–430 CrossRef CAS.
- G. Rauret, J.-F. López-Sánchez, A. Sahuquillo, E. Barahona, M. Lachica, A. M. Ure, C. M. Davidson, A. Gómez, D. Lück, J. Bacon, M. Yli-Halla, H. Muntau and Ph. Quevauviller, J. Environ. Monit., 2000, 2, 228–233 RSC.
- M. Pueyo, J. Sastre, E. Hernández, M. Vidal, J. F. López-Sánchez and G. Rauret, J. Environ. Qual., 2003, 32, 2054–2066 CrossRef CAS.
- F. Zucconi, M. Forte, A. Monaco and M. de Bertoldi, BioCycle, 1981, 22, 27–29 Search PubMed.
- M. J. Wierzbicka and J. Obidzinska, Plant Sci. (Lucknow, India), 1998, 137, 155–171 Search PubMed.
- A. Masaguer and M. Benito, in Compostaje, ed. J. Moreno and R. Moral, Mundi-Prensa, Madrid, 2008, pp. 285–304 Search PubMed.
- R. Paradelo, A. B. Moldes, B. Prieto, R. G. Sandu and M. T. Barral, Compost Sci. Util., 2010, 18, 22–31 Search PubMed.
- Ministry of Agriculture, Spanish Official Bulletin, 2005, vol. 171, pp. 25592–25669 Search PubMed.
- L. F. Díaz and G. M. Savage, in Compost Science and Technology, ed. L. F. Díaz, M. de Bertoldi, W. Bidlingmaier and E. Stentiford, Elsevier, Amsterdam, 2007, pp. 49–66 Search PubMed.
- P. Krauss, R. Blessing and U. Korherr, in Compost: Production, Quality and Use, ed. M. de Bertoldi, Elsevier, London, 1987, pp. 254–265 Search PubMed.
- G. Petruzzelli, I. Scymura, L. Lubrano and B. Pezzarossa, Environ. Technol. Lett., 1989, 10, 521–526 CAS.
- A. Veeken and B. Hamelers, Sci. Total Environ., 2002, 300, 87–98 CrossRef CAS.
- R. Paradelo, A. B. Moldes and M. T. Barral, Waste Manage. Res., 2009, 27, 46–51 Search PubMed.
- V. K. Sharma, M. Canditelli, F. Fortuna and G. Cornacchia, Energy Convers. Manage., 1997, 38, 453–478 CrossRef CAS.
- J. Doublet, C. Francou, J. P. Pétraud, M. F. Dignac, M. Poitrenaud and S. Houot, Bioresour. Technol., 2010, 101, 1254–1262 CrossRef CAS.
- C. García, T. Hernandez, F. Costa and M. Ayuso, Suelo Planta, 1991, 1, 1–13 Search PubMed.
- Y. Liu, L. Ma, Y. Li and L. Zheng, Chemosphere, 2007, 67, 1025–1032 CrossRef CAS.
- M. Farrell and D. L. Jones, Bioresour. Technol., 2009, 100, 4423–4432 CrossRef CAS.
- L. Leita and M. de Nobili, J. Environ. Qual., 1991, 20, 73–78 CrossRef CAS.
- S. Amir, M. Hafidi, G. Merlina and J. C. Revel, Chemosphere, 2005, 59, 801–810 CrossRef CAS.
- T. Pare, H. Dinel and M. Schnitzer, Biol. Fertil. Soils, 1999, 29, 31–37 CrossRef CAS.
- N. W. Menzies, M. J. Donn and P. M. Koppitke, Environ. Pollut., 2007, 145, 121–130 CrossRef CAS.
- S. P. Singh, F. M. Tack and M. G. Verloo, Water, Air, Soil Pollut., 1998, 102, 313–328 CrossRef CAS.
- M. H. Wong and A. D. Bradshaw, New Phytol., 1982, 91, 255–261 CrossRef CAS.
- K.-Y. Chiang, H.-J. Huang and C.-H. Chang, J. Environ. Eng. Manage., 2007, 17, 249–256 Search PubMed.
- A. V. Filgueiras, I. Lavilla and C. Bendicho, J. Environ. Monit., 2002, 4, 823–857 RSC.
- P. L. Genevini, V. Mezzanote and A. Garbarino, Waste Manage. Res., 1987, 5, 501–511 Search PubMed.
- X.-T. He, T. J. Logan and S. J. Traina, J. Environ. Qual., 1995, 24, 543–552 CAS.
- F. J. Stevenson, Humus Chemistry: Genesis, Composition, Reactions, John Wiley & Sons, New York, 1982 Search PubMed.
- J. W. C. Wong and A. Selvam, Chemosphere, 2006, 63, 980–986 CrossRef CAS.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844 CrossRef CAS.
- W. Salomons and U. Forstner, Metals in the Hydrocycle, Springer-Verlag, Berlin, 1984 Search PubMed.
- M. Meguellati, D. Robbe, P. Marchandise and M. Astruc, in Proceedings of the International Conference Heavy Metals in the Environment, ed. G. Miller, CEP Consultants, Heidelberg, 1983, p. 1090 Search PubMed.
- Y.-Y. Long, L.-F. Hu, C.-R. Fang, Y.-Y. Wu and D.-S. Shen, Microchem. J., 2009, 91, 1–5 CrossRef CAS.
- S. V. Vassilev, C. Braekman-Danheux and P. Laurent, Fuel Process. Technol., 1999, 59, 95–134 CrossRef CAS.
- Z. S. Ahnstrom and D. R. Parker, Soil Sci. Soc. Am. J., 1999, 63, 1650–1658 CAS.
- L. J. Sikora and V. Yakovchenko, Soil Sci. Soc. Am. J., 1996, 60, 1401–1404 CrossRef CAS.
- B. Gabrielle, J. da Silveira, S. Houot and J. Michelin, Agric., Ecosyst. Environ., 2005, 110, 289–299 CrossRef.
- T. K. Hartz, J. P. Mitchell and C. Giannini, HortScience, 2000, 35, 209–212 Search PubMed.
- J. R. Mantovani, M. E. Ferreira, M. C. P. da Cruz, J. C. Barbosa and A. C. Freiria, Rev. Bras. Cienc. Solo, 2006, 30, 677–684 Search PubMed.
- E. R. Emino and P. R. Warman, Compost Sci. Util., 2004, 12, 342–348 Search PubMed.
- R. Paradelo, A. B. Moldes, M. Rodríguez and M. T. Barral, Cienc. Tecnol. Aliment., 2008, 6, 143–151 Search PubMed.
- R. Paradelo, A. Villada, D. González and M. T. Barral, Fresenius Environ. Bull., 2010, 19, 956–962 CAS.
| This journal is © The Royal Society of Chemistry 2011 |