Pathways for methanol steam reforming involving adsorbed formaldehyde and hydroxyl intermediates on Cu(111): density functional theory studies
Abstract
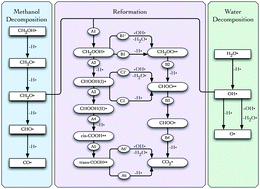
Plane-wave density functional theory calculations have been carried out to explore possible pathways in

 Please wait while we load your content...
Please wait while we load your content...