Brønsted acid-catalyzed enantioselective Friedländer condensations: achiral amine promoter plays crucial role in the stereocontrol†‡
Abstract
A highly enantioselective Friedländer condensation has been established by using chiral Brønsted acids in combination with achiral
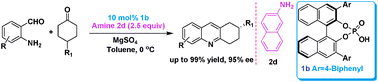
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...