Efficient and scalable serial extraction of DNA and RNA from frozen tissue samples†‡
Lucy
Mathot
,
Monica
Lindman
and
Tobias
Sjöblom
*
Department of Genetics and Pathology, Uppsala University, SE-751 85 Uppsala, Sweden. E-mail: tobias.sjoblom@genpat.uu.se
First published on 23rd November 2010
Abstract
Advances in cancer genomics have created a demand for scalable sample processing. We here present a process for serial extraction of nucleic acids from the same frozen tissue sample based on magnetic silica particles. The process is automation friendly with high recoveries of pure DNA and RNA suitable for analysis.
The landscapes of somatic mutations in common human cancers consist of few frequently mutated genes along with several recently discovered genes of low mutation prevalence.1 Somatic mutation analyses for discovery, diagnostic, and response predictive purposes are therefore likely to entail analyses of sets of genes, rather than individual genes, in each patient. Such analyses are preferably based on fresh-frozen tissue samples, as the length and quality of nucleic acids recovered is superior to that obtained from formaldehyde-fixed paraffin embedded materials. Ongoing cancer genome sequencing efforts, along with the increasing use of response predictive biomarkers based on somatic mutations, have created a need for high quality DNA and RNA from thousands of fresh-frozen patient tumours and matched normal tissues.2
In tumor biobanks, tissue embedding in water soluble glycols and resin before freezing represents a convenient way to preserve morphology and biomolecule integrity over time.3Extraction of DNA or RNA from such materials can be performed with chaotropic agents and silica based solid supports.4 However, there is a lack of scalable technology for consecutive extraction of DNA and RNA from frozen tissues and cell pellets.5 The criteria for such a procedure are (1) suitability for extraction of any tissue type; (2) ability to extract resin-embedded frozen tissues; (3) automation friendliness with uninterrupted parallel processing of samples with no centrifugation or filtration steps; (4) recovery of long strands of high quality DNA as evidenced by performance in downstream applications; and (5) recovery of high quality total RNA as measured by performance in downstream applications.6
We present here a simple procedure whereby frozen cell pellets or sections of OCT-embedded frozen tissues are lysed by grinding in chaotropic salt solution and differentially captured onto siliceous solid supports.7 As DNA competes with RNA for binding to silica-based solid supports, we reasoned that prior recovery of DNA would facilitate subsequent recovery of RNA (Fig. S1 ESI‡). Therefore, DNA is first recovered from the lysate using magnetic silica beads with selectivity for DNA over RNA that are subsequently washed and eluted. Total RNA is then recovered after a second incubation of the lysate with magnetic silica beads, washing and elution (Fig. 1).8
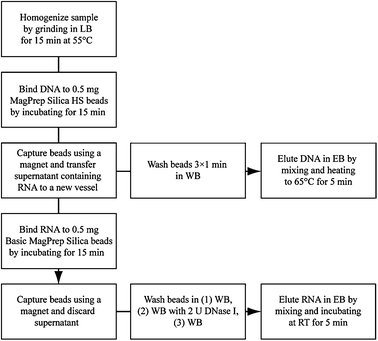 | ||
| Fig. 1 Procedure for sequential extraction of DNA and RNA from frozen tissues. Lysis Buffer (LB): 7 M guanidine HCl, 50 mM Tris pH 7, 1 mg mL−1proteinase K, 2% Tween 20; Wash Buffer (WB): 10 mM Tris-HCl pH 6.5; Elution Buffer (EB): 10 mM Tris-HCl pH 9, 1 mM EDTA. | ||
DNA from three 10 μm thick OCT embedded frozen tissue sections from breast, colon, spleen, tonsil, or frozen bone marrow cell pellets (3 × 106cells) was extracted as described in Fig. 1 (for a detailed description, see ESI‡). For assessment of performance in DNA isolation, 0.5 mg of each bead [MagPrep Silica HS (Merck), SiMag (Chemicell), Magnesil (Promega), MagsiDNA (Magnamedics), S1.0 and S1.0-COOH (Mobitec) and Accubead (Bioneer)] were used. Sample matched control DNA was extracted from OCT embedded frozen tissue by overnight proteinase K digestion followed by phenol chloroform extraction and ethanol precipitation. Spectrophotometrical quantification of DNA was performed using a NanoDrop instrument. Real time PCR quantification of the human LINE1 repeat element was carried out, followed by assessment of DNA fragment length by electrophoretic separation in a 0.4% agarose gel with a High Range DNA ladder as size standard. PCR amplification and sequencing of exons 1 to 7 of the PRPS1 gene was carried out as described in the ESI.‡
First, we screened commercially available magnetic silica beads with different surface chemistries for their respective abilities to extract genomic DNA of high quality from frozen tissue lysates (Fig. 2A). Several solid supports showed marked differences in eluted DNA quantities as measured by optical density and functionally by ability to template PCR (Fig. 2). For example, the recovery of high quality DNA, as defined by amplification efficiency in the polymerase chain reaction (PCR), was 6-fold higher with MagPrep Silica HS beads than the recovery with S1.0-COOH beads. In contrast, the difference in recovery measured by optical density was only 3-fold. Physical differences between beads of the same surface chemistries also affect DNA recovery, as shown by the >10-fold difference between MagPrep beads and other SiOH-derivatized beads. When compared to other beads, DNA yields with MagPrep Silica HS were consistently higher as measured by real time PCR amplification of LINE1 elements (U-test, P = 2 × 10−6).
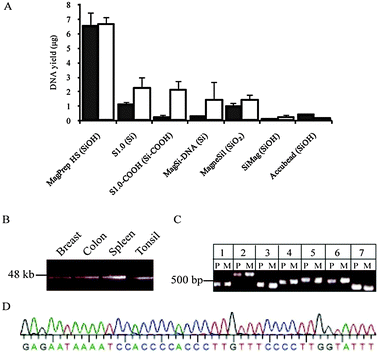 | ||
| Fig. 2 Recovery of high quality genomic DNA from frozen tissue samples using MagPrep HS magnetic silica particles. (A) DNA recovery from lysates of 3 sections of 10 μm OCT embedded frozen spleen employing different silica beads was quantified by spectrophotometry (open bars) and real time PCR amplification of LINE1 elements (solid bars). Mean and SD of 3 independent experiments. (B) Fragment lengths of DNA extracted from breast, colon, spleen, and tonsil using MagPrep HS particles. (C) PCR amplification of protein encoding exons of PRPS1. The 7 protein-encoding exons of PRPS1 were amplified by PCR in genomic DNA obtained from normal colon by phenol-chloroform extraction (P), and in genomic DNA obtained using MagPrep HS particles (M). (D) Capillary sequencing trace of PRPS1 exon 7 in genomic DNA obtained from normal colon using MagPrep HS particles. | ||
We therefore selected MagPrep Silica HS particles for further studies as they consistently produced the highest DNA yields and quality. When applied in serial purification from tissue sections, the yield of total DNA was in the range 1–10 μg. Sizing by electrophoresis demonstrated that the recovered DNA fragments were up to 48 kb in length, similar to DNA from tissues extracted by phenol–chloroform (Fig. 2B and Fig. S2A ESI‡). The gDNA extracted was found to consist solely of long fragments (See Fig. S3 ESI‡).
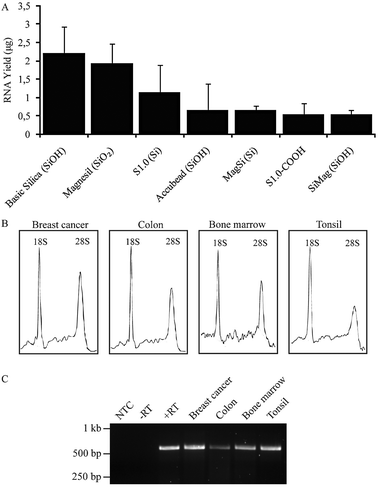 | ||
| Fig. 3 Recovery of high quality total RNA with MagPrep Basic magnetic silica particles after DNA purification . (A) Total RNA yields after binding of total RNA from frozen tonsil tissue to siliceous beads of different types, followed by washing and elution. Mean and SD of 3 experiments. (B) Electrophoretic separation of total RNA samples extracted from breast cancer, colon, bone marrow and tonsil. (C) Reverse transcription-coupled PCR amplification of ACTB in serially purified RNA from lysates of breast cancer, colon, frozen bone marrow cell pellets and tonsil. NTC, non-template control; −RT, no reverse transcriptase; +RT, positive control. | ||
We then assessed the DNA quality by PCR coupled Sanger sequencing of all seven exons of the PRPS1 gene using genomic DNA extracted from colon as template. All primer pairs yielded expected PCR products with similar efficiency in genomic DNA prepared from frozen colon sections using MagPrep Silica HS as in DNA prepared from frozen colon of the same patient by phenol–chloroform extraction (Fig. 2C). Dye terminator sequencing of the PCR products amplified from the MagPrep Silica HS purified template yielded high quality sequences with 99% PHRED20 bases in the protein-encoding regions of interest (Fig. 2D). No RNA contamination of the eluted DNA was observed in reverse transcription-PCR amplification of ACTB using eluted DNA as template (Fig. S2B ESI‡). For massively parallel paired end sequencing of genomes, it is desirable that the starting material contains long molecules of genomic DNA.9 The DNA amount, length and purity obtained with MagPrep HS purification should enable successful use of tissue-derived nucleic acids in such applications.
Secondly, we devised a simple bead-based process for isolation of total RNA from the remaining cleared lysate after DNA recovery. As the MagPrep Silica HS particles are known to preferentially bind DNA over RNA, we screened MagPrep Silica Basic particles which have similar affinity for DNA as for RNA along with the beads assessed in DNA extraction above for their ability to extract total RNA from tissue in the same lysis, wash, and elution buffers employed for DNA purification (Fig. 3A). When compared to other beads, RNA yields with MagPrep Basic Silica were consistently higher (U-test, P = 3 × 10−2).
The RNA integrity and concentration was determined in a Bioanalyzer instrument (Agilent) using an RNA 6000 Nano kit. 5 ng of RNA was reverse transcribed to cDNA and used to PCR amplify ACTB (see ESI‡). Using MagPrep Silica Basic particles, the RNA recovery was 2–2.5 μg. The integrity of a control total RNA sample extracted using MagPrep Silica Basic particles was not affected by the procedure (Fig. S4 ESI‡). When applied in serial purification from tissue sections, the yield of total RNA is in the range 1–2.5 μg. RNA of high integrity from lysates of frozen breast tumour, colon, tonsil and bone marrow frozen cell pellets was obtained after serial extraction of DNA and RNA (Fig. 3B). This was used as a template in reverse transcription-coupled PCR amplification of ACTB (Fig. 3C). In all cases, the expected β-actin products were observed, demonstrating that the total RNA fractions recovered were of sufficient purity and quality for downstream applications.
Several procedures for column-based serial recovery of DNA and RNA from blood and tissues have been devised and commercialized. To the best of our knowledge, this represents the first such serial nucleic acid extraction procedure intended for automated use with frozen biobanked tissue materials. Multiple aspects of this procedure render it suitable for automation: all separations can be achieved in 96- or 384-well format with a plate magnet, the total number of liquid reagents is kept as low as six, the binding of nucleic acids to MagPrep beads is controlled solely by pH and does not require the use of alcohol in the wash steps, and the concordance between spectrophotometric and functional quantification of the eluted genomic DNA also enables use of the former to estimate the latter by concentration measurements in a simple plate reader.
We envision that this procedure, when combined with automated sample storage systems, will greatly enhance large scale biomolecule extraction from normal tissues, solid tissues and leukaemias stored in biobanks. Taken together, advantages of the serial DNA and RNA extraction procedure presented here include (1) recovery of different nucleic acids from the same cells of a normal tissue or tumour; (2) reduction of labour and reagents by a common lysis step and use of the same buffers in wash and elution; (3) recovery of long DNA strands suitable for rearrangement analyses and whole genome sequencing; (4) increased RNA capture efficiency due to prior removal of DNA from the same sample lysate; (5) solid phases that obviate the need for ethanol in wash solutions, thereby avoiding alcohol contamination in eluates that may inhibit downstream reactions; and (6) applicability to a wide range of input materials.
This study was supported by Uppsala/Umeå Comprehensive Cancer Consortium (U-CAN), the Swedish Cancer Foundation and the Swedish Children's Cancer Foundation. We thank Sean Hooper for assistance with statistical analyses.
Notes and references
- T. Sjöblom, S. Jones, L. D. Wood, W. Parsons, J. Lin, T. D. Marber, D. Mandelker, R. J. Leary, J. Ptak, N. Silliman, S. Szabo, P. Buckhaults, C. Farrell, P. Meeh, S. D. Markowitz, J. Willis, D. Dawson, J. Willson, A. Gazdar, J. Hartigan, L. Wu, C. Liu, G. Parmigiani, B. Park, K. E. Bachman, N. Papadopoulos, B. Vogelstein, K. Kinzler and V. Velculescu, Science, 2006, 314, 268–274 CrossRef.
- http://cancergenome.nih.gov .
- P. Micke, M. Ohshima, S. Tahmasebpoor, Z. P. Ren, A. Östman, F. Pontén and J. Botling, Lab. Invest., 2006, 86, 202–211 Search PubMed; M. Schmitt, K. Mengele, E. Schueren, F. Sweep, J. A. Foekens, N. Brünner, J. Laabs, A. Malik and N. Harbeck, Eur. J. Cancer, 2007, 43, 835–844 CrossRef CAS.
- P. Krieg, E. Amtmann and G. Sauer, Anal. Biochem., 1983, 134, 288–294 CrossRef CAS; B. Vogelstein and D. Gillespie, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 615–619 CrossRef CAS; K. Kang, J. Choi, J. H. Nam, S. C. Lee, K. J. Kim, S. W. Lee and J. H. Chang, J. Phys. Chem. B, 2009, 113, 536–543 CrossRef CAS; S. Berensmeier, Appl. Microbiol. Biotechnol., 2006, 73, 495–504 CrossRef CAS.
- K. Obata, O. Segawa, M. Yakabe, Y. Ishida, T. Kuroita, K. Ikeda, B. Kawakami, Y. Kawamura, M. Yohda, T. Matsunaga and H. Tajima, J. Biosci. Bioeng., 2001, 91, 500–503 CrossRef CAS; M. K. Hourfar, U. Michelsen, M. Schmidt, A. Berger, E. Seifried and W. K. Roth, Clin. Chem., 2005, 51, 1217–1222 CrossRef CAS.
- R. Boom, C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Werthem van Dillen and J. van der Noordaa, J. Clin. Microbiol., 1990, 28, 495–503 CAS.
- F. J. Castellino and R. Barker, Biochemistry, 1968, 7, 4135–4138 CrossRef CAS.
- P. R. Levison, S. E. Badger, J. Dennis, P. Hathi, M. J. Davies, I. J. Bruce and D. Schimkat, J. Chromatogr., A, 1998, 816, 107–111 CrossRef CAS; K. A. Melzak, C. S. Sherwood, R. F. B. Turner and C. A. Haynes, J. Colloid Interface Sci., 1996, 181, 635–644 CrossRef CAS; P. R. Levison, J. W. Dennis, K. D. Jones, R. W. Philpott, S. L. Taylor and V. Grimm, Clin. Chem., 1998, 44, 2060–2061 CAS.
- S. Lasken and M. Egholm, Trends Biotechnol., 2003, 21, 531–535 CrossRef.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Materials, methods, supplementary data and references. See DOI: 10.1039/c0cc02248a |
| This journal is © The Royal Society of Chemistry 2011 |
