Cis-Trans isomerisation of azobenzenes studied by laser-coupled NMR spectroscopy and DFT calculations†‡
Abstract
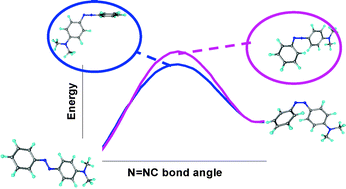
In a combined experimental and computational study of a
- This article is part of the themed collection: In honour of Jan Verhoeven

 Please wait while we load your content...
Please wait while we load your content...