In situ growth of gold nanoparticles on latent fingerprints—from forensic applications to inkjet printed nanoparticle patterns†
Irshad
Hussain
*ab,
Syed Zajif
Hussain
a,
Habib-ur-Rehman
a,
Ayesha
Ihsan
b,
Asma
Rehman
b,
Zafar M.
Khalid
b,
Mathias
Brust
c and
Andrew I.
Cooper
c
aLUMS School of Science & Engineering (SSE), D.H.A., Lahore Cantt, 54792, Pakistan
bNational Institute for Biotechnology & Genetic Engineering (NIBGE), Jhang Road, Faisalabad, Pakistan. E-mail: ihussain@lums.edu.pk
cDepartment of Chemistry, University of Liverpool, L69 7Zd, Liverpool, UK
First published on 20th October 2010
Abstract
Latent fingerprints are made visible in a single step by in situ growth of gold nanoparticles on ridge patterns. The chemicals, among the essential components of human sweat, found responsible for the formation and assembly of gold nanoparticles are screened and used as ink to write invisible patterns, using common ball pen and inkjet printer, which are then developed by selectively growing gold nanoparticles by soaking them in gold salt solution.
Latent fingerprints consist of a complex mixture of natural biological secretions and contaminants from the environment. These are typically used for personal identification and their detection still ranks among the best sources of evidence for crime investigation. Several methods for the detection of such latent fingerprints are already available1–13 but the integration of nanotechnology with these detection methods offers several advantages in terms of improved selectivity, enhanced contrast, and higher sensitivity.14–36 An increasing trend in the use of metal nanoparticles and quantum dots has recently been observed to develop latent fingerprints.15–17,21,23,26,27,31–33,36,37 These preformed nanoparticles are usually either adsorbed electrostatically or their surface is specifically modified to generate affinity for the components of sweat.15,23,31,36 Multimetal deposition of metal nanoparticles onto latent fingerprints has been examined extensively to visualize latent fingerprints and usually consists of several cumbersome steps.32,33 In the first step, functionalized nanoparticles are adsorbed on the fingerprints followed by treatment with various physical developers to improve the contrast.32,33 An important advantage of using gold nanoparticles for this purpose has been the long-term storage of developed fingerprints due to their inert nature.
We now propose a simple and one-step method for the detection of latent fingerprints by in situ production of gold nanoparticles from solutions of gold salts, exploiting the components of human sweat as the sole reducing and binding agents. Interestingly, the components of sweat (lecithin and KI) which were found to be responsible for the formation of gold nanoparticles were then used to develop a bio-ink to write invisible phrases and circuit patterns on materials such as paper and a polymer using a common ball pen and an inkjet printer. Soaking of these invisible patterns in gold solution resulted in the selective formation of gold nanoparticles rendering the writing and the patterns clearly visible. This simple method of generating gold nanoparticle patterns on inkjet printed biochemicals has great potential to offer an attractive alternative to conventional photolithography for the production of electronic devices.38–48 Among various other obvious advantages, this method may also offer the flexibility, low cost and easier production of nanoparticle patterns on a large scale for flexible electronics.
Sebum-rich (sebaceous) fingerprints were obtained from the volunteers by rubbing their fingers, washed with soap and ethanol, onto their foreheads and stamping them onto A4 paper or Whatman cellulose filter paper strips. The paper strips bearing the sebaceous prints were immersed in an aqueous solution of gold chloride (HAuCl4·3H2O, 1 mM) at room temperature. The fingerprints started to become visible after about one hour with the development of a red colour, a characteristic colour of the gold nanoparticles (3–30 nm) which results from their surface plasmon resonance absorption. The colour of these prints changed from red to purple with time due to their surface plasmon absorbance and coupling as the particles grow and more and more particles are formed (please see ESI†). Generally the latent fingerprints become clearly visible within 4 hours (Fig. 1) and, once formed, these fingerprints retain their colour for at least 6 months (the maximum storage time tested so far). We expect these developed fingerprints to retain their colour for years due to the inert nature of gold. Field emission scanning electron micrographs (please see ESI†) of these coloured fingerprints confirmed the presence of gold nanoparticles (15–20 nm) on the ridge patterns. Similar patterns, though less clear, can also be developed on plastic surfaces, i.e., petri dish (please see ESI†).
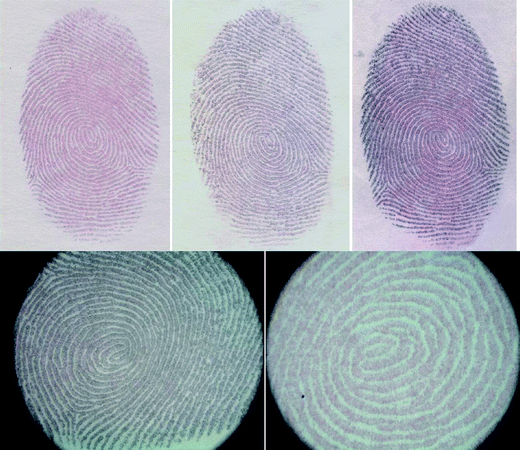 | ||
| Fig. 1 Optical images of latent fingerprints after development by in situ growth of gold nanoparticles on ridge patterns on paper strips. | ||
We then set out to understand which chemicals among the sebaceous/eccrine secretions could be responsible for the reduction of ionic gold to form gold nanoparticles. We developed a simple spot test to screen various possible chemicals and their combinations for their ability to form gold nanoparticles at room temperature (Fig. 2). The list of the chemicals and their combinations tested for this purpose is given as a table in Fig. 2. About 20 µL of aqueous solution/suspension (100 mM each) of the listed chemicals and their combinations were spotted on a Whatman cellulose filter paper, dried at room temperature and then soaked in 1 mM gold salt (HAuCl4·3H2O) solution for 4 hours. Using this simple strategy, the chemicals or combination of chemicals responsible for the formation of gold nanoparticles were detected by observing the development of coloured spots. The development of red or purple colour indicated the formation of gold nanoparticles. Fig. 2 shows the response of all the tested chemicals and it is clear that lecithin in particular has the ability to reduce ionic gold (Au3+) to gold nanoparticles on cellulose paper in combination with various other chemicals at room temperature. An important observation was the ability of KI alone to concentrate gold salt in the region where it is spotted or in combination with other chemicals (note the light yellowish colour where KI is spotted), and also to improve the rate of formation of gold nanoparticle as indicated by the rapid appearance of red or purple spots. Lecithin and its combination with KI resulted in the formation of clear red spots and this combination was thus selected for the subsequent studies.
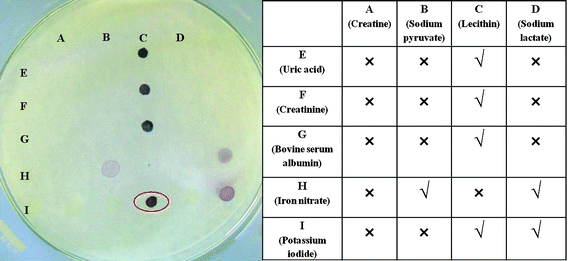 | ||
| Fig. 2 A spot test to screen various chemicals found in human sweat for their ability to reduce ionic gold to gold nanoparticles at room temperature. The tested chemicals and their response are summarized in the table. | ||
Knowing that lecithin can be used as a good reducing agent for the production of gold nanoparticles, we then examined its potential for the synthesis of suspended gold nanoparticles in the absence of any other reducing/stabilizing agents. Although room temperature reduction of ionic gold was slower, the addition of a warm lecithin aqueous suspension to boiling HAuCl4·3H2O resulted in the formation of purple coloured gold nanoparticles suspension with a characteristic surface plasmon absorption band at 542 nm. The addition of KI, however, improved the rate of reduction of ionic gold and gold nanoparticles with fairly similar optical properties were obtained at room temperature. In both cases, the gold nanoparticles range in size from 15 to 25 nm as shown in the transmission electron micrograph (Fig. 3) which was obtained using a scanning transmission field emission electron microscope (JSM 7500F).
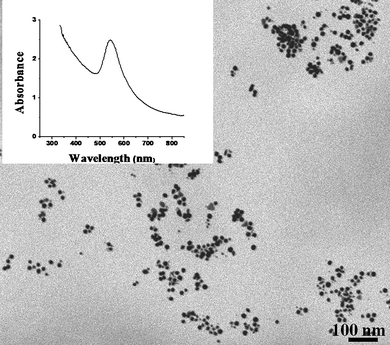 | ||
| Fig. 3 Field emission scanning electron micrograph of gold nanoparticles formed using lecithin as a sole reducing/stabilizing agent. The inset shows their UV-visible absorption spectrum. | ||
Inspired by the room temperature reduction capability of a mixture of lecithin and KI, we set out to use this combination as a bio-ink to produce gold nanoparticles patterns on hand-written and inkjet printed prototypes. As a proof of principle, we rubbed our institutional stamps against the foreheads of a few volunteers and then stamped on a typical office paper (A4 sheet, 80 g m−2). As expected, soaking the stamped paper strips in aqueous solution of HAuCl4·3H2O resulted in the formation of gold nanoparticles rendering the stamped patterns clearly visible (please see ESI†). We then removed the ink completely from a common ball pen and re-filled it with a viscous aqueous suspension of bio-ink (mixture of lecithin and KI). This bio-ink was then used to write invisible phrases and patterns which were subsequently developed and made visible by the formation of gold nanoparticles after soaking the latent images in an aqueous solution of HAuCl4·3H2O (please see ESI†). Most of these patterns were associated with a varying degree of background associated with non-specific adsorption which depended on the quality of paper, presumably due to the formation of gold nanoparticles via reduction by cellulose and other components in the paper. Using strips of a typical office paper (A4 sheet, 80 g m−2) and Whatman cellulose filter paper, however, resulted in a significant reduction in the background thus improving contrast and making the patterns clearly visible.
Finally, we used the suspension of a mixture of lecithin and KI as a bio-ink to fill the empty cartridges of an inkjet printer and this was used to print invisible text and circuit patterns onto high quality paper. The invisible printed text and circuit patterns became clearly visible after soaking the latent images in an aqueous solution of HAuCl4·3H2O (Fig. 4a and b). Our initial studies show that these gold nanoparticle patterns are not conductive due to insufficient connectivity of nanoparticles but we are now trying to improve the loading and the connectivity of the gold nanoparticles by incubating these patterns in dilute solutions of alkanedithiols followed by the development of another layer of preformed gold nanoparticles. In addition, we are also working on the electroless deposition of silver onto these patterns to make them conductive which could facilitate the mass production of flexible electronics. We are also exploring other substrates which can be used to print such patterns in addition to determining their potential resolution. We believe this simple strategy may be useful for forensic applications as well as offering an approach for the mass production of nanoparticle-based patterns for applications in optics, electronics and sensing.
 | ||
| Fig. 4 In situ growth of gold nanoparticles on invisible bio-ink (lecithin + KI) printed text (A) and circuit patterns using inkjet printer (B). | ||
Acknowledgements
We thank National Commission on Nanoscience and Technology (NCNST) and Ministry of Science and Technology (MoST), Govt of Pakistan, for financial support to initiate nano-biotechnology research at NIBGE, Faisalabad. I.H. also thanks LUMS School of Science & Engineering (SSE), Lahore, Pakistan for start-up research funds.Notes and references
- J. Almog, A. Hirshfeld and J. T. Klug, J. Forensic Sci., 1982, 27, 912–917 CAS.
- J. Almog, V. G. Sears, E. Springer, D. F. Hewlett, S. Walker, S. Wiesner, R. Lidor and E. Bahar, J. Forensic Sci., 2000, 45, 538–544 CAS.
- S. Bell, Annu. Rev. Anal. Chem., 2009, 2, 297–319 Search PubMed.
- A. L. Beresford and A. R. Hillman, Anal. Chem., 2010, 82, 483–486 CrossRef CAS.
- Q. Chen, W. T. Kerk, A. M. Soutar and X. T. Zeng, Appl. Clay Sci., 2009, 44, 156–160 CrossRef CAS.
- A. J. Goddard, A. R. Hillman and J. W. Bond, J. Forensic Sci., 2010, 55, 58–65 CrossRef.
- P. Hazarika, S. M. Jickells and D. A. Russell, Analyst, 2009, 134, 93–96 RSC.
- D. R. Ifa, N. E. Manicke, A. L. Dill and G. Cooks, Science, 2008, 321, 805 CrossRef CAS.
- S. M. Jickells, Meas. Control, 2008, 41, 243–247 Search PubMed.
- P. F. Kelly, R. S. P. King and R. J. Mortimer, Chem. Commun., 2008, 6111–6113 RSC.
- F. Rowell, K. Hudson and J. Seviour, Analyst, 2009, 134, 701–707 RSC.
- D. J. Spedding, Nature, 1971, 229, 123–124 CrossRef CAS.
- M. Zhang and H. H. Girault, Analyst, 2009, 134, 25–30 RSC.
- J. Almog and H. Glasner, J. Forensic Sci., 2010, 55, 215–220 CrossRef CAS.
- A. Becue, C. Champod and P. Margot, Forensic Sci. Int., 2007, 168, 169–176 CrossRef CAS.
- A. Becue, S. Moret, C. Champod and P. Margot, Forensic Sci. Int., 2009, 191, 36–41 CrossRef CAS.
- K. H. Cheng, J. Ajimo and W. Chen, J. Nanosci. Nanotechnol., 2008, 8, 1170–1173 CAS.
- M. J. Choi, A. M. McDonagh, P. Maynard and C. Roux, Forensic Sci. Int., 2008, 179, 87–97 CrossRef CAS.
- M. J. Choi, T. Smoother, A. A. Martin, A. M. McDonagh, P. J. Maynard, C. Lennard and C. Roux, Forensic Sci. Int., 2007, 173, 154–160 CrossRef CAS.
- J. Dilag, H. Kobus and A. V. Ellis, Forensic Sci. Int., 2009, 187, 97–102 CrossRef CAS.
- D. Gao, F. Li, J. Song, X. Xu, Q. Zhang and L. Niu, Talanta, 2009, 80, 479–483 CrossRef CAS.
- M. Grouchko, A. Kamyshny and S. Magdassi, J. Mater. Chem., 2009, 19, 3057–3062 RSC.
- P. Hazarika, S. M. Jickells, K. Wolff and D. A. Russell, Angew. Chem., Int. Ed., 2008, 47, 10167–10170 CrossRef CAS.
- D. Huang, F. Liao, S. Molesa, D. Redinger and V. Subramanian, J. Electrochem. Soc., 2003, 150, G412–G417 CrossRef CAS.
- G. Kwak, W. E. Lee, W. H. Kim and H. Lee, Chem. Commun., 2009, 2112–2114 RSC.
- C. McCormick, MRS Bull., 2007, 32, 526.
- E. R. Menzel, S. M. Savoy, S. J. Ulvick, K. H. Cheng, R. H. Murdock and M. R. Sudduth, J. Forensic Sci., 2000, 45, 545–551 CAS.
- B. T. Nguyen, J. E. Gautrot, M. T. Nguyen and X. X. Zhu, J. Mater. Chem., 2007, 17, 1725–1730 RSC.
- S. Roy, J. Phys. D: Appl. Phys., 2007, 40, R413–R426 CrossRef CAS.
- B. H. Ryu, Y. Choi, H. S. Park, J. H. Byun, K. Kong, J. O. Lee and H. Chang, Colloids Surf., A, 2005, 270, 345–351 CrossRef.
- M. Sametband, I. Shweky, U. Banin, D. Mandler and J. Almog, Chem. Commun., 2007, 1142–1144 RSC.
- B. Schnetz and P. Margot, Forensic Sci. Int., 2001, 118, 21–28 CrossRef CAS.
- E. Stauffer, A. Becue, K. V. Singh, K. R. Thampi, C. Champod and P. Margot, Forensic Sci. Int., 2007, 168, e5–e9 CrossRef CAS.
- X. F. Tang, Z. G. Yang and W. J. Wang, Colloids Surf., A, 2010, 360, 99–104 CrossRef CAS.
- B. J. Theaker, K. E. Hudson and F. J. Rowell, Forensic Sci. Int., 2008, 174, 26–34 CrossRef CAS.
- O. S. Wolfheis, Angew. Chem., Int. Ed., 2009, 48, 2268–2269.
- H. W. Tang, W. Lu, C. M. Che and K. M. Ng, Anal. Chem., 2010, 82, 1589–1593 CrossRef CAS.
- S. M. Bidoki, J. Nouri and A. A. Heidari, J. Micromech. Microeng., 2010, 20, 055023 CrossRef.
- M. Boberl, M. V. Kovalenko, S. Gamerith, E. J. W. List and W. Heiss, Adv. Mater., 2007, 19, 3574–3578 CrossRef.
- E. Chow, J. Herrmann, C. S. Barton, B. Raguse and L. Wieczorek, Anal. Chim. Acta, 2009, 632, 135–142 CrossRef CAS.
- E. Fisslthaler, A. Blumel, K. Landfester, U. Scherf and E. J. W. List, Soft Matter, 2008, 4, 2448–2453 RSC.
- S. Gamerith, A. Klug, H. Scheiber, U. Scherf, E. Moderegger and E. J. W. List, Adv. Funct. Mater., 2007, 17, 3111–3118 CrossRef CAS.
- S. F. Jahn, T. Blaudeck, R. R. Baumann, A. Jakob, P. Ecorchard, T. Ruffer, H. Lang and P. Schmidt, Chem. Mater., 2010, 22, 3067–3071 CrossRef CAS.
- M. Layani, M. Gruchko, O. Milo, I. Balberg, D. Azulay and S. Magdassi, ACS Nano, 2009, 3, 3537–3542 CrossRef CAS.
- J. U. Park, J. H. Lee, U. Paik, Y. Lu and J. A. Rogers, Nano Lett., 2008, 8, 4210–4216 CrossRef CAS.
- J. Perelaer, A. W. M. de Laat, C. E. Hendriks and U. S. Schubert, J. Mater. Chem., 2008, 18, 3209–3215 RSC.
- J. D. Swartz, L. F. Deravi and D. W. Wright, Adv. Funct. Mater., 2010, 20, 1488–1492 CrossRef CAS.
- T. H. J. van Osch, J. Perelaer, A. W. M. de Laat and U. S. Schubert, Adv. Mater., 2008, 20, 343–345 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure and some supporting images. See DOI: 10.1039/c0nr00593b |
| This journal is © The Royal Society of Chemistry 2010 |
