Superhydrophilicity-assisted preparation of transparent and visible light activated N-doped titania film
Qing Chi
Xu
a,
Diana V.
Wellia
a,
Rose
Amal
b,
Dai Wei
Liao
c,
Say Chye Joachim
Loo
d and
Timothy Thatt Yang
Tan
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459. E-mail: tytan@ntu.edu.sg; Fax: +65-67911761; Tel: +65-63168829
bSchool of Chemical Sciences and Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
cDepartment of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, People's Republic of China
dSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798
First published on 15th May 2010
Abstract
A novel and environmental friendly method was developed to prepare transparent, uniform, crack-free and visible light activated nitrogen doped (N-doped) titania thin films without the use of organic Ti precursors and organic solvents. The N-doped titania films were prepared from heating aqueous peroxotitanate thin films deposited uniformly on superhydrophilic uncoated glass substrates. The pure glass substrates were superhydrophilic after being heated at 500 °C for 1 h. Nitrogen concentrations in the titania films were adjusted by changing the amount of ammonia solution. The optimal photocatalytic activity of the N-doped titania films was about 14 times higher than that of a commercial self-cleaning glass under the same visible light illumination. The current reported preparative technique is generally applicable for the preparation of other thin films.
Introduction
Titania films on glass slides have potential applications in solar cells,1 for self-cleaning2 and decontamination applications.3 However, a pure titania film can only be activated under UV light, which prevents their widespread application. A variety of methods had been devised to extend the optical absorption range to the visible light region, by doping with transition metals (Cr, Fe, Mn, V)4–7 and nonmetal atoms (N, S, C).8–11 N-doped titania has attracted great interest due to its viable application under visible light.8Various methods have been used to prepare N-doped titania films, such as LP-MOCVD,12 reactive magnetron sputtering,13 AP-CVD,14 radio-frequency (RF) sputtering,15 thermal treatment under an N2 or NH3 atmosphere,16 atomic layer deposition,17 pulsed laser deposition18 and sol–gel methods,19etc. Among all the reported techniques for titania film preparation, expensive equipment and controlled environment are usually required. Moreover, these techniques consume a great deal of energy and are difficult to scale up.20 The sol–gel method is the most widely used as it presents many advantages such as the use of very simple equipment and low capital investment, and the ability to control the microstructure and density of the thin films. However, the solvent used for coating is mainly organic in nature, which may potentially be an environmental pollutant. Also, common sol–gel methods often involve organic titanium compound and organic solvents which are expensive. Organic titanium compound undergoes strong hydrolysis and hence forms white precipitates when it comes into contact with atmospheric water. Special equipment that creates a water-free environment will therefore be needed in order to prevent such a rapid hydrolysis reaction.
To avoid these drawbacks, aqueous peroxotitanate solutions with different viscosities have been employed to prepare titania films.21–24 Gao et al. prepared a titania film by floating pure glass on the surface of an aqueous peroxotitanate solution for an extended period of 12–120 h.22,23 However, the solution became turbid after soaking for about 15 min and the coating formed was not uniform and transparent. Some cracks were also observed in the as-prepared titania film. Ge et al.21 and Yuan et al.24 prepared titania film by dipping glass slides in aqueous peroxotitanate solution. However, due to the high surface tension of the water-based solution, aqueous peroxotitanate solution could not disperse on the glass uniformly and there were also some cracks in the titania films. In view of these, it is challenging to form uniform and transparent titania thin films on smooth glass substrates without cracks using typical coating techniques such as dip-coating and spin-coating.
To date, great efforts have been dedicated to prepare superhydrophilic films for self-cleaning applications. An aqueous solution can disperse uniformly on a superhydrophilic surface and form a uniform thin film. However, the preparation of a uniform and transparent thin film by exploiting this superhydrophilic property has not been reported. In addition, to the best of our knowledge, there is no report in the open literature on transparent N-doped titania films prepared by using aqueous peroxotitanate solution as a Ti source. In this work, we report a novel method for the preparation of transparent, uniform, crack-free and visible light activated N-doped titania thin films by using aqueous peroxotitanate solution (PTA) as precursor and exploiting the superhydrophilicity of pure glass substrates. This method is “green” as no organic solvent and organic Ti complex are involved. Since water is a commonly used, stable, economical and environmental friendly solvent, this synthetic approach can be easily generalized into a technology for the preparation of other thin films by exploiting superhydrophilic substrates and aqueous precursors. We have prepared Bi2WO6 and cation-doped TiO2 thin films using similar strategy and will communicate our findings in future works.
Experimental details
Reagents
Titanium(IV) chloride (purity > 99%) and ammonia solution (25%) were purchased from Merck. Hydrogen peroxide (30%) was purchased from AnalaR. The chemicals were used without further purification.Preparation of aqueous peroxotitanate solution (PTA)
The method for preparation of PTA solution was reported elsewhere.23 In a typical preparation, 3.6 ml of TiCl4 was added drop-wise into a 300 ml distilled water in an ice-water bath with strong magnetic stirring. After 30 min, the pH of the solution was adjusted to 7, 8, 9, 10 and 10.5 by drop-wise addition of diluted ammonia solution. After stirring for 24 h, the obtained white precipitates were filtered and washed thoroughly with distilled water repeatedly until Cl− was not detected. Thereafter the precipitates were ultrasonic dispersed in 80 ml distilled water. H2O2 (28 ml) was added drop-wise into this mixture under stirring. The concentration of titania of the resulting transparent yellow solution was adjusted to 2.0 wt%.Preparation of N-doped titania films
Glass slides were heated at 500 °C for 1 h, cooled down to room temperature and used within 0.5 h. Aqeous peroxotitanate solution was then dip-coated on the freshly heated glass slides. Finally, the resultant films were heated in air at 500 °C for 1 h. The N-doped TiO2 films prepared at different pH of 7, 8, 9, 10 and 10.5 were labeled as N–TiO2–7, N–TiO2–8, N–TiO2–9, N–TiO2–10 and N–TiO2–10.5, respectively. The scheme for the preparation of N doped titania films from aqueous peroxotitanate solution is illustrated in Fig. 1.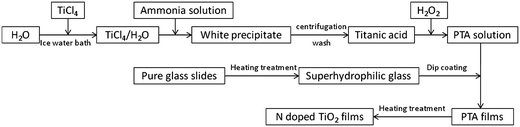 | ||
| Fig. 1 Scheme for the preparation of N-doped titania films from aqueous peroxotitanate solution. | ||
Characterization
X-Ray diffraction analysis (XRD) was carried out by a Philips PW1010 X-ray diffractometer with Cu-Kα radiation. An XRD pattern was recorded with a scan step of 1° min−1 (2θ) in the range from 10° to 70°. X-Ray photoelectron spectroscopy (XPS) analysis was carried out with a PHI Quantum 2000 Scanning ESCA Micro-probe equipment (Physical Electronics, MN, USA) using monochromatic Al-Kα radiation. The X-ray beam diameter was 100 μm, and the pass energy was 29.35 eV for the sample. The binding energy was calibrated with respect to C (1s) at 284.6 eV. Surface morphologies and thicknesses of N-doped titania films were evaluated by JEOL field emission electron microscope JSM-6700F. UV-Vis spectra of films were obtained using a UV-Visible spectrophotometer (Shimadzu). The sessile drop method was used for contact angle measurements with a FTA200 Dynamic Contact Angle analyzer.Photocatalytic tests
For the evaluation of photocatalytic activities of the N-doped titania films, one 300 W halogen lamp held at 15 cm from the sample with a 420 nm UV filter (JB420) was used as the visible light source. The change in the stearic acid layer thickness was monitored by measuring the infrared absorption spectrum with a FTIR instrument (Digilab FTS 3100). The absorbance at 2917 cm−1 was converted to a thickness on the basis of an earlier observation that an absorbance intensity of 0.01 corresponds to a thickness of 12.5 nm.25 Commercial Pilkington Activ™ self-cleaning glass was used for comparison.Results and discussion
Hydroxyl group generation in silica glass is a consequence of reactions of silica glass with thermally loaded molecules, such as H2O and H2 molecules present in the glass upon heat treatment.26 It was reported that OH groups were formed from reactions of molecular water diffused into silica glass with the glass network or network-bound groups.27–29 Nuccio et al. claimed that intrinsic generation processes of OH groups in synthetic silica glass also existed, involving reactions between the glass network and chemical species in the material.26 Yokomachi et al. also reported that the exposure of silica glass to high temperatures increased the OH-groups concentration by nearly fourfold due to weakened hydrogen bonds.30 All these works report an increase in hydroxyl groups concentration on silica glass after thermal treatment. The wettability of titania film can be also enhanced with the increase of absorbed hydroxyl group.31 However, the contact angles and superhydrophilicity of heat treated silica glass have not been reported. Fig. 2 shows the contact angle (CA) of pure glass slide measured within 1 h, after being heated at different temperatures for 1 h and cooled down to room temperature. The CA decreased with the increase of heating temperature, i.e. CA decreased to ∼2.5° after being heated at 500 °C while the CA of the glass slide heated at 650 °C is almost 0°. The freshly heated glass slides are temporarily superhydrophilic even when they are not coated with any materials. This temporal superhydrophilic property of the heat treated silica glass slide is attributed to the increase of surface Si–OH concentration and the decrease of surface contaminant. Fig. 2 (inset) shows the contact angle of the heat treated glass slide after being left in ambient condition for different durations (h). It can be seen that CA increased rapidly to 52° after 24 h. Therefore, the superhydrophilicity of heat-treated pure glass is temporal and should be used before the CA increases to beyond 5°. This increase in CA may be due to the decrease of hydroxyl concentration or the absorption of contaminant on the silica glass surface.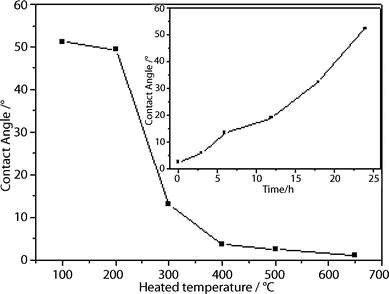 | ||
| Fig. 2 Contact angle of pure glass after being heated at different temperatures. (Inset: Variation of contact angle of heated glass (500 °C) with time after being left in ambient conditions). | ||
The images of glass slides with and without heat treatment after being dipped into aqueous peroxotitanate solution followed by subsequent formation of N-doped titania film are shown in Fig. 3. Due to the high surface tension of the water-based solution, aqueous peroxotitanate solution could not disperse uniformly on the glass substrate. From Fig. 3A, we can see only certain parts of the glass surface were deposited with peroxotitanate and droplets were found on the glass surface. This resulted in non-uniform coating. For the freshly heat treated glass substrate (Fig. 3B), a thin aqueous peroxotitanate solution was observed to be coated uniformly on the glass substrate. The glass substrate appears slightly opaque due to a thin film of peroxotitanate solution coated on the surface. Fig. 3C and Figure 3D show the images of N–TiO2–10 film coated on pure glass with and without heat treatment, respectively. It is obvious that the titania coating in Fig. 3D is not uniform while the titania coating in Fig. 3C is uniform, transparent, and a little yellow due to the doping of N atoms in the titania film.
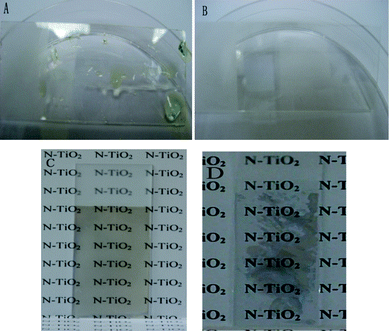 | ||
| Fig. 3 Images of pure glass substrates (A) without and (B) with heat treatment after being dipped into PTA aqueous solution; (C) coated with transparent N–TiO2–10 film on temporary superhydrophilic glass; (D) coated with N-doped titania film on glass without heat treatment. | ||
The crystalline phase and particle size of N-doped titania were determined by XRD (Fig. 4). The distinctive peaks at 2θ = 25.3°, 38.0°, 48.1°, 53.8°, 54.8° and 62.8° are attributed to the anatase titania structure, which indicates that all the N-doped titania thin films mainly consist of the anatase phase. The crystal sizes of N–TiO2–7, N–TiO2–8, N–TiO2–9, N–TiO2–10 and N–TiO2–10.5 are approximately 17.0 nm as determined by Scherrer's equation.
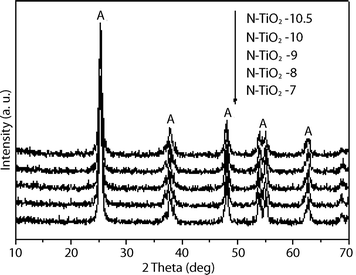 | ||
| Fig. 4 XRD pattern of N-doped titania prepared at different pH conditions. | ||
The FTIR spectra of the peroxotitanate, prepared at different pH conditions, before (Fig. 5a) and after heat treatment (Fig. 5b) are presented. In Fig. 5a, the absorption bands at 900 cm−1 are attributed to the stretching vibrations of the peroxo band (O–O).24 The wide bands at 3100–3700 cm−1 are attributed to the vibration of adsorbed water and the bands at 1400 cm−1 are assigned to the stretching vibrations of the N–H bonds in NH3, which provides evidence of the presence of NH3 in Ti complex.32 The NH3 in the Ti complex is regarded as a nitrogen source for the N-doped titania.33,34Fig. 5b shows the FTIR of peroxotitanate after calcination. It is obvious that the O–O bands at 900 cm−1 and the N–H bands at 1400 cm−1 disappeared, which provides evidence of the decomposition of peroxotitanate and NH3. A new weak peak appears at 1387 cm−1, which is assigned to hyponitrite.35,36
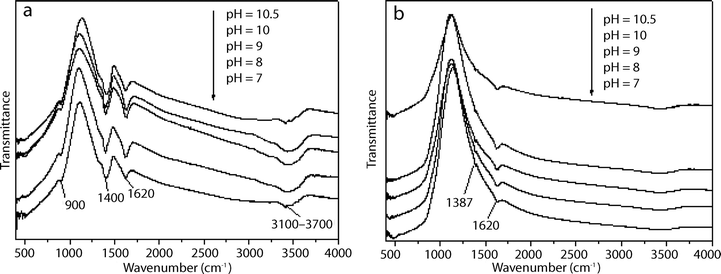 | ||
| Fig. 5 FTIR spectra of the peroxotitanate coating on glass substrates prepared at different pH values (a) before and (b) after being heated at 500 °C for 1 h (N-doped titania film). | ||
Sun et al.33 reported that the hydrolysis of TiCl4, with the addition of ammonia solution, formed a Ti complex with the formula [Ti(H2O)a(NH3)b(OH)cCld](4−c−d) (where a + b + c + d = 6). The Ti complex formed seems to be affected by the presence of ions such as ammonium, hydroxide and chloride. Similarly, Cheng et al.37 reported that the hydrolysis of TiCl4 with the addition of KOH solution formed [Ti(OH)nClm]2− complex (where m + n = 6). The value of m decreased with the increase in pH and the decrease in [Cl−]. In our case, with increasing pH, the concentrations of NH3 and OH− increase, which probably led to the increase of the b and c values, and the decrease of the d value in the [Ti(H2O)a(NH3)b(OH)cCld](4−c−d) complex. Sun et al. suggested that the NH3 in the Ti complex were close to the Ti atoms, which might lead to an easy doping process.33 NH3 in the Ti complex would be decomposed during the crystal transformation process, which render the N atoms easier to be incorporated into the titania.34 The doped nitrogen concentration is dependent on the amount of NH3 in the Ti complex.33 With the increase in pH and hence NH3 concentration, the concentration of NH3 in the Ti complex increases and the doping nitrogen concentration increases.
Fig. 6 shows N 1s XPS spectra for the N-doped titania samples. The peaks at 400.0 eV are found in all the N-doped titania films and the intensities increased with the increase of pH value. The XPS signal at around 400 eV has been a subject of controversy in the identification of nitrogen species in the study of N-doped titania. Many reports have suggested that the signal at 400 eV is attributed to NO species adsorbed on crystallite surface.33 Sakthivel et al.35 and Navio et al.36 reported that the signal at around 400 eV was attributed to the presence of hyponitrite. Qiu et al. assigned the signal at 400 eV to the nitrogen incorporated into the titania lattice.34 However, they unanimously reported that the peak at 400 eV was crucial for a visible light response. The nitrogen concentrations in the samples N–TiO2–7, N–TiO2–8, N–TiO2–9, N–TiO2–10 and N–TiO2–10.5 are determined to be 0.08 at%, 0.20 at%, 0.26 at%, 0.78 at% and 0.96 at%, respectively. The color of the N-doped titania changes from pale yellow to yellow with the increase of pH value, which is consistent with the increase of nitrogen concentration determined by XPS.
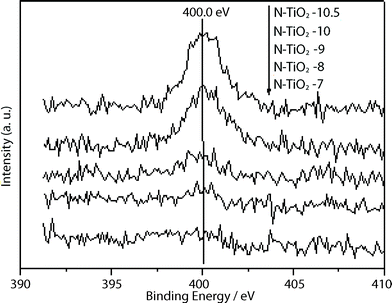 | ||
| Fig. 6 N 1s XPS spectra of N-doped titania films. | ||
The optical absorption spectra of those N-doped titania films are shown in Fig. 7. All the N-doped titania films exhibit absorption in the visible light region. The absorption intensity gradually increased with the increase of pH value due to the increase of nitrogen doping concentration in titania.
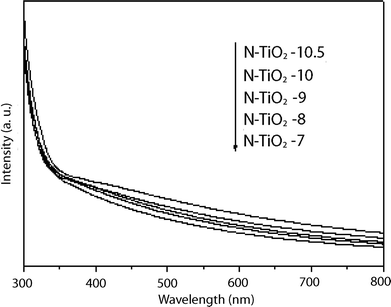 | ||
| Fig. 7 UV-Visible diffuse reflectance spectra of N-doped titania films prepared at different pH conditions. | ||
The morphologies of the N–TiO2–10 film were further characterized by FESEM (Fig. 8). Titania films prepared by other methods20,22,24 using aqueous peroxotitanate solution exhibited some cracks. However, no cracks were found in the films prepared using the current method (Fig. 8a). This is attributed to uniform coating of peroxotitanate on the superhydrophilic pure glass with high –OH groups concentration. Fig. 8b shows that the particle size of N-doped titania is around 17–20 nm, which is consistent with that obtained from XRD. The morphology of other N-doped titania films, which are not shown here, were similar to that of the N–TiO2–10 film.
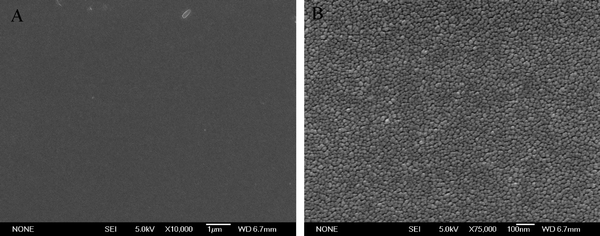 | ||
| Fig. 8 FESEM images for N–TiO2–10 film (A) at low and (B) high magnifications. | ||
The photocatalytic activity of the N-doped titania films were evaluated and compared with a commercial titania self-cleaning glass by monitoring the degradation of stearic acid under visible light. Fig. 9a shows the IR spectra of stearic acid on N–TiO2–10 film. The intensity of stearic acid signal decreased significantly after 24 h of visible light illumination. The photocatalytic activities of the various thin films under visible light illumination were evaluated and shown in Fig. 9b. The photoactivities were evaluated based on the thickness (in nm) of stearic acid degraded after 24 h of illumination.38 The photocatalytic activities of N-doped titania films increased with the increase of pH (increase of ammonia concentration) until pH 10. As suggested in the preceding paragraph, this could be attributed to the increase of N-doping concentration, which may lead to an improvement of visible light photoactivity. The thickness of stearic acid degraded for the N–TiO2–10 was 11.3 nm, which was 9 times higher than that of N–TiO2–7 and 14 times higher than that of a commercial self-cleaning glass. At the highest ammonia concentration (pH = 10.5), the thickness of stearic acid degraded decreases to 7.5 nm. Higher dopant concentrations may lead to greater recombination rate and lower photocatalytic activity.39
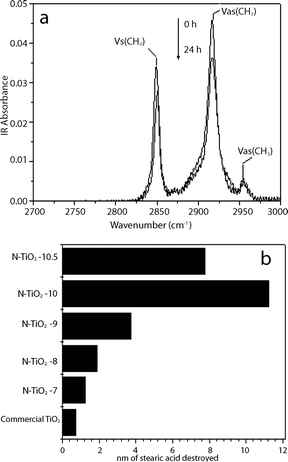 | ||
| Fig. 9 (a) Evolution of the IR absorbance spectra (N–TiO2–10) under visible light illumination; (b) Photocatalytic activities of N-doped titania films prepared at different pH under visible light illumination for 24 h. | ||
Conclusion
We have developed a “green” method for the preparation of N-doped titania films by exploiting the superhydrophilicity of freshly heated pure glass and using organic compound-free PTA solution as the Ti source. The N-doped titania films were uniform, transparent and crack-free. All the N-doped titania films prepared were photocatalytic under visible light illumination. The photocatalytic activity of N–TiO2–10 was about 14 times higher than that of a commercial self-cleaning glass under the same visible light illumination. In addition, this method can be developed into a general coating technology for the preparation of other types of thin films which involves aqueous solutions and superhydrophilic surfaces.Acknowledgements
Financial support from Nanyang Technological University AcRF Tier 1 RG29/07 is gratefully acknowledged.References
- A. Mishira, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS.
- X. Feng, J. Zhai and L. Jiang, Angew. Chem., Int. Ed., 2005, 44, 5115 CrossRef CAS.
- T. Kawahara, Y. Konishi, H. Tada, N. Tohge, J. Nishii and S. Ito, Angew. Chem., Int. Ed., 2002, 41, 2811 CrossRef CAS.
- S. Klosek and D. Raftery, J. Phys. Chem. B, 2001, 105, 2815 CrossRef CAS.
- M. Anpo and M. Takeuchi, J. Catal., 2003, 216, 505 CrossRef CAS.
- A. K. Ghosh and H. P. Maruska, J. Electrochem. Soc., 1977, 124, 1516 CrossRef CAS.
- W. Y. Choi, A. Termin and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13669 CrossRef.
- R. Asashi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS.
- S. U. M. Khan, M. Al-shahry and W. B. Ingler Jr., Science, 2002, 297, 2243 CrossRef CAS.
- T. Umebayashi, T. Yamaki, H. Itoh and K. Asai, Appl. Phys. Lett., 2002, 81, 454 CrossRef CAS.
- Q. C. Xu, D. V. Wellia, M. A. SK, K. H. Lim, Joachim, S. C. Loo, D. W. Liao, R. Amal Timothy and T. Y. Tan, J. Photochem. Photobiol. A: Chem., 2010 DOI:10.1016/j.jphotochem.2009.12.017.
- J. Guillot, F. Fabreguette, L. Imhoff, O. Heintz, M. C. Marco de Lucas, M. Sacilotti, B. Domenichini and S. Bourgeois, Appl. Surf. Sci., 2001, 177, 268 CrossRef CAS.
- J. M. Mwabora, T. Lindgren, E. Avendano, T. F. Jaramillo, J. Lu, S.-E. Lindquist and C.-G. Granqvist, J. Phys. Chem. B, 2004, 108, 20193 CrossRef CAS.
- N. Sato, M. Matsuda, M. Yoshinaga, T. Nakamura, S. Sato and A. Muramatsu, Top. Catal., 2009, 52, 1592 CrossRef CAS.
- Y. W. Sakai, K. Obata, K. Hashimoto and H. Irie, Vacuum, 2008, 83, 683 CrossRef CAS.
- L. Mi, P. Xu and P.-N. Wang, Appl. Surf. Sci., 2008, 255, 2574 CrossRef CAS.
- H. E. Chen, W.-J. Lee, C.-M. Hsu, M.-H. Hon and C.-L. Huang, Electrochem. Solid-State Lett., 2008, 11, D81 CrossRef.
- L. Zhao, Q. Jiang and J. Lian, Appl. Surf. Sci., 2008, 254, 4620 CrossRef CAS.
- J. Ananpattarachai, P. Kajitvichyanukul and S. Seraphin, J. Hazard. Mater., 2009, 168, 253 CrossRef CAS.
- Y. F. Gao, Y. Masuda, Z. Peng, T. Yonezawa and K. Koumoto, J. Mater. Chem., 2003, 13, 608 RSC.
- L. Ge, M. X. Xu, M. Sun and H. B. Fang, J. Sol-Gel Sci. Technol., 2006, 38, 47 CrossRef CAS.
- Y. F. Gao, Y. Masuda and K. Koumoto, Langmuir, 2004, 20, 3188 CrossRef CAS.
- Y. F. Gao, Y. Masuda and K. Koumoto, Chem. Mater., 2004, 16, 1062 CrossRef CAS.
- Z. F. Yuan, B. Li, J. L. Zhang, C. Xu and J. J. Ke, J. Sol-Gel Sci. Technol., 2006, 39, 249 CrossRef CAS.
- V. Pore, M. Ritala, M. Leskela, S. Areva, M. Jarn and J. Jarnstrom, J. Mater. Chem., 2007, 17, 1361 RSC.
- L. Nuccio, S. Agnello and R. Boscaino, Appl. Phys. Lett., 2008, 93, 151906 CrossRef.
- K. Kajihara, M. Hirano, L. Skuja and H. Hosono, J. Appl. Phys., 2005, 98, 043515 CrossRef.
- J. W. Lee and M. Tomozawa, J. Non-Cryst. Solids, 2007, 353, 4633 CrossRef CAS.
- A. Oehler and M. Tomozawa, J. Non-Cryst. Solids, 2004, 347, 211 CrossRef CAS.
- Y. Yokomachi, R. Tohmon, K. Nagasawa and Y. Ohki, J. Non-Cryst. Solids, 1987, 95–96, 663 CrossRef CAS.
- J. C. Yu, J. Yu, H. Y. Tang and L. Zhang, J. Mater. Chem., 2002, 12, 81 RSC.
- Y. Xie, Y. Li and X. Zhao, J. Mol. Catal. A: Chem., 2007, 277, 119 CrossRef CAS.
- H. Sun, Y. Bai, H. Liu, W. Jin, N. Xu, G. Chen and B. Xu, J. Phys. Chem. C, 2008, 112, 13304 CrossRef CAS.
- X. Qiu, Y. Zhao and C. Burda, Adv. Mater., 2007, 19, 3995 CrossRef CAS.
- S. Sakthivel, M. Janczarek and H. Kisch, J. Phys. Chem. B, 2004, 108, 19384 CrossRef CAS.
- J. A. Navio, C. Cerrillos and C. Real, Surf. Interface Anal., 1996, 24, 355 CrossRef CAS.
- H. Cheng, J. Ma, Z. Zhao and L. Qi, Chem. Mater., 1995, 7, 663 CrossRef CAS.
- X. Chen, Y. Lou, A. C. S. Samia, C. Burda and J. L. Gole, Adv. Funct. Mater., 2005, 15, 41 CrossRef CAS.
- H. Irie, Y. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 5483 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
