The effects of the hydrophobic environment on proton mobility in perfluorosulfonic acid systems: an ab initio molecular dynamics study†
Abstract
Model systems of
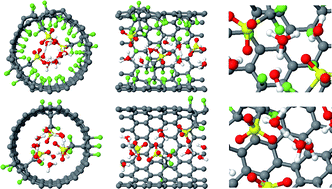
- This article is part of the themed collection: Proton Transport for Fuel Cells

 Please wait while we load your content...
Please wait while we load your content...