Syngas production via high-temperature steam/CO2 co-electrolysis: an economic assessment
Abstract
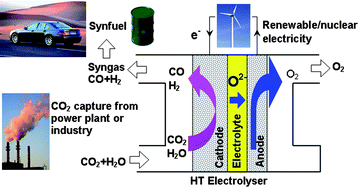
Although it is not yet technologically mature, the high-temperature steam/CO2 co-electrolysis process offers potentially a feasible and environmentally benign way to convert

 Please wait while we load your content...
Please wait while we load your content...