Activation of a water molecule using a mononuclear Mn complex: from Mn-aquo, to Mn-hydroxo, to Mn-oxyl via charge compensation†
Abstract
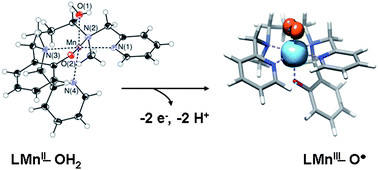
Activation of a water molecule by the electrochemical oxidation of a Mn-aquo complex accompanied by the loss of protons is reported. The sequential (2 × 1 electron/1 proton) and direct (2 electron/2 proton) proton-coupled electrochemical oxidation of a non-porphyrinic six-coordinated Mn(II)OH2 complex into a mononuclear Mn(O) complex is described. The intermediate Mn(III)OH2 and Mn(III)OH complexes are electrochemically prepared and analysed. Complete deprotonation of the coordinated water molecule in the Mn(O) complex is confirmed by electrochemical data while the analysis of EXAFS data reveals a gradual shortening of an Mn–O bond upon oxidation from Mn(II)OH2 to Mn(III)OH and Mn(O). Reactivity experiments, DFT calculations and XANES pre-edge features provide strong evidence that the bonding in Mn(O) is best characterized by a Mn(III)-oxyl description. Such oxyl species could play a crucial role in natural and artificial water splitting reactions. We provide here a synthetic example for such species, obtained by electrochemical activation of a water ligand.

 Please wait while we load your content...
Please wait while we load your content...