Emulsion-assisted synthesis of monodisperse binary metal nanoparticles†
Zhen
Yin
,
Ding
Ma
* and
Xinhe
Bao
*
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, The Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: dma@dicp.ac.cn; xhbao@dicp.ac.cn; Fax: +86-0411-84694447; Tel: +86-0411-84379253
First published on 11th January 2010
Abstract
An easy-to-scale-up multiphase emulsion-assisted synthetic strategy based on a ternary metal precursor/surfactant/ethylene glycol system has been established to prepare monodispersed bimetallic nanoparticles with designated size and composition; the nanoparticles thus prepared are easily separable from the reaction mixture.
Bimetallic nanoparticles have drawn increasing interest over the past decade because of their novel optoelectronic, magnetic, and catalytic properties.1 Specifically, Pd-based bimetallic nanoparticles are one of the most important catalysts due to their unique and different properties from those of the monometallic Pd nanoparticles.2 Though various methods have been developed to control the size, shape and composition of the composite nanoparticles,3 most of these methods need to use expensive organometallic precursors which are highly toxic and not easy to control during reactions due to the very high volatility of these precursors, especially in a bimetallic system.
For the preparation of Pd-based bimetallic nanoparticles, various methods have been reported, including thermal decomposition of organometallic precursors,4 alcohol reduction,5 reducing agent reduction such as amine–borane complexes6 or ascorbic acid,7 and the microemulsion method.8 However, these methods either require expensive organometallic precursors or cannot be scaled up. A classical/efficient method to prepare metal nanoparticles is the ethylene glycol (EG) reduction process and its variations.9 When it is extended to bimetallic systems, the size distribution of the products turns out to be very broad, and the shape and composition hard to manipulate due to the difficulty in controlling nucleation and growth of nanoparticles.10 Here we report a novel multiple-phase emulsion-assisted synthetic strategy to prepare a variety of Pd-based bimetallic nanoparticles with a narrow size distribution. This process uses metallic acetates as the metal precursors. With oleic acid and oleylamine dispersed in EG, an emulsion-like system was created for the fabrication of bimetallic nanoparticles with a narrow size distribution.
Scheme 1 shows the general protocol for the fabrication of monodispersed bimetallic nanoparticles (details in the ESI†). Oleic acid (OA) and oleylamine (OAm) were dispersed in the EG to form a turbid solution under vigorous stirring. Subsequently, certain metal precursor solutions (in this case, Pd(CH3COO)2 in acetone and Au(CH3COO)3 in deionized water) were added into the above-mentioned dispersion simultaneously. An emulsion system was thus obtained, with the metal precursor solutions as the dispersed phase and OA/OAm acting as surfactants. The mixture was heated to 393 K and kept at this temperature for 60 min. The nucleation/crystal growth occurred at this stage, leading to the de-emulsification of the system, which was signaled by the appearance of dark colored precipitates. Finally, an amount of hexane was added into the mixture to extract the colloidal metal nanoparticles. Then, the colloidal metal nanoparticles were transferred into the hexane layer (hydrophobic phase) and finally concentrated by evaporation, washed several times with excess ethanol until the supernatant was colorless. The final product was easily re-dispersed in hexane. The yield of a typical bimetallic PdAu nanoparticles synthesis reaction is about 65%.
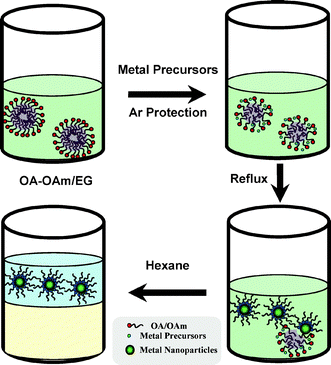 | ||
| Scheme 1 Schematic representation of bimetallic nanoparticle synthesis in the emulsion-assisted multiphase system. | ||
The PdAu system will be discussed below in detail as an example. The X-ray diffraction patterns (XRD) of the obtained samples are very similar to those of either Au or Pd with a face-centered structure, as shown in Fig. 1. A slight shift (about 0.3°) of Au to higher angles in the peak position can be observed, since the metallic Pd crystals have a smaller lattice than Au (see the colored bars in Fig. 1C which indicate the standard diffraction peaks of Pd and Au). Energy dispersive X-ray (EDX) spectrum analysis with high resolution transmission electron microscopy (HRTEM) performed on a single nanoparticle chosen randomly shows that the structures contained both Pd and Au with a mean molar ratio of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 when the molar ratio of metal precursor is 1
69 when the molar ratio of metal precursor is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2 and Table S2 in ESI†), which is consistent with the molar ratios determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Table S3 in ESI†). HRTEM images (inset of Fig. 1B) show that a typical nanoparticle is mainly of the same lattice spacings, with a lattice spacing of 2.31 Å, corresponding to the interplanar distance of the (111) planes of fcc Au or Pd. From the intensity and position of the surface plasmon resonance (SPR) peak observed in the ultraviolet-visible absorption (UV-vis) spectra for bimetallic PdAu nanoparticles (Fig. 1D), we confirmed further the incorporation of Pd atoms into the Au nanoparticles. These results indicate the formation of a bimetallic phase of Au and Pd.
1 (Fig. S2 and Table S2 in ESI†), which is consistent with the molar ratios determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Table S3 in ESI†). HRTEM images (inset of Fig. 1B) show that a typical nanoparticle is mainly of the same lattice spacings, with a lattice spacing of 2.31 Å, corresponding to the interplanar distance of the (111) planes of fcc Au or Pd. From the intensity and position of the surface plasmon resonance (SPR) peak observed in the ultraviolet-visible absorption (UV-vis) spectra for bimetallic PdAu nanoparticles (Fig. 1D), we confirmed further the incorporation of Pd atoms into the Au nanoparticles. These results indicate the formation of a bimetallic phase of Au and Pd.
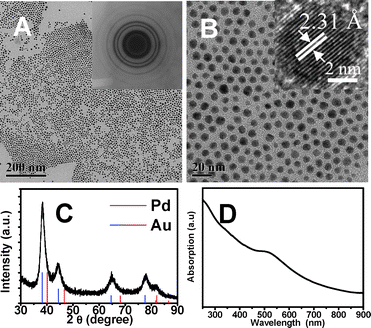 | ||
| Fig. 1 (A, B) TEM images of as-synthesized PdAu nanoparticles at different magnification (particle size of 7.4 nm). Insert images show an electron diffraction image and a HRTEM of the PdAu nanoparticles, respectively; (C) XRD pattern of the product and (D) UV-vis spectrum of the nanoparticles. | ||
The size and shape of the synthesized bimetallic PdAu nanoparticles were investigated using TEM. Fig. 1 shows representative images of PdAu bimetallic nanoparticles at different magnifications. The average particle size is 7.4 nm (with a narrow size distribution <7%), in accordance with the size calculated from the XRD results by the Scherrer equation.
To understand the formation mechanism of the bimetallic nanoparticles, we utilized UV-vis spectroscopy to trace the reaction process of PdAu bimetallic nanoparticle fabrication.10b The samples were recovered from the reaction mixture at different temperatures and time intervals. After extracting with hexane, each sample was diluted to a fixed volume before taking UV-vis measurements (see the picture of the sample solutions in Fig. S3†). Fig. 2 shows UV-vis absorption spectra of the samples dispersed in hexane. The strong absorption at 520 nm was observed when the reaction mixture was heated to 373 K, which arises from the excitation of the SPR of the Au nanoparticles2d,11 and indicates that the nucleation of gold clusters happened at a temperature up to 373 K. This adsorption keeps its shape until 383 K, and becomes weak and broadens gradually (from 393 K, and those with more reaction time at 393 K), which is believed to be caused by changes in the composition of the products. With further reaction, only a weak feature at around 510 nm can be observed in the UV-vis spectrum. With the above results, we proposed that Au nucleates firstly to Au nanoclusters and grows with the elevating temperature. The growing particles would be hydrophobically surface-ligated by OA/OAm, and would reside within the OA/OAm liquid phase. Subsequently Pd atoms would deposit and nucleate on/in the Au nanoclusters, with growth being moderated by the surface ligands. Finally, bimetallic PdAu nanoparticles result as the reaction goes on.12
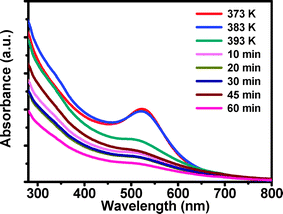 | ||
| Fig. 2 UV-vis spectra of the products taken out of the reaction mixture at different reaction stages. | ||
In our study, this method has also been extended to the synthesis of other bimetallic systems. Fig. 3 shows TEM images of PdAg, PdCu, AuPt and AuAg nanoparticles synthesized using the same method. It is clear that monodispersed metal nanoparticles with narrow size distributions were obtained, showing the adaptability of this method to various systems (XRD patterns, UV-vis and EDX spectra of the PdAg and PdCu nanoparticles are shown in Fig. S5†). As each product has a very narrow size distribution, no size-selection procedure was required if monodispersed bimetallic nanoparticles are expected. Moreover, the size and composition of binary nanoparticles can be tuned by varying reaction parameters such as the time, and the molar ratio of two metal precursors or the surfactant to metal precursors. Detailed research is still in progress and will be reported in a separate publication.
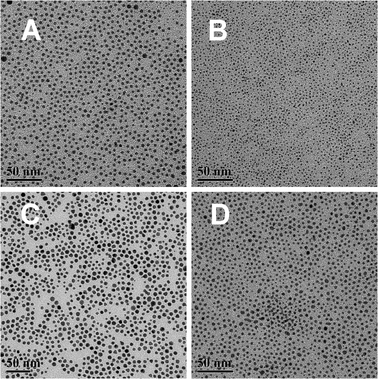 | ||
| Fig. 3 TEM images of as-synthesized bimetallic nanoparticles: (A) 4.2 nm PdAg; (B) 1.9 nm PdCu; (C) 6.3 nm AuPt; (D) 4.0 nm AuAg. The scale bars are 50 nm. All images were acquired without a size selection process. | ||
In the study, we noticed that the bimetallic nanoparticles easily self-organize into 2D and 3D superlattices because of the uniform structural features of the particles in size and shape. Fig. S7† shows TEM images of the close-packed superlattices of PdAg and AuAg nanoparticles, which in turn shows the efficiency of using this simple method to prepare monodispersed bimetallic nanoparticles.
It should also be addressed that the current method is a big step forward from the conventional ethylene glycol (EG) reduction method. The nanoparticles prepared in the conventional EG reduction system need a complicated washing and separation process due to the high viscosity of glycol and its strong affinity for nanoparticles. Instead, as shown in Scheme 1, the advantage of the current method is that the final bimetallic nanoparticles are hydrophobic due to surface-modification by a surfactant layer, hence a post-treatment based on extraction with a non-polar solvent can be adopted (Fig. S1 in ESI†).13 In other words, the final bimetallic nanoparticles can be easily extracted into the hexane phase and recovered from the system. This process, once fully developed, will make the mass production of bimetallic nanoparticles possible.
In summary, a facile method has been developed for the synthesis of a series of bicomponent metallic nanoparticles with a narrow size distribution. In principle, the process can be easily scaled up because of its straightforward synthetic strategy. More importantly, the as-prepared nanoparticles could be easily separated from the reaction mixture. This process was proven to be adaptable to the synthesis of a series of bicomponent metallic nanoparticles with narrow size distribution.
We thank the National Natural Science Foundation of China (20773121) for support. D.M. thanks the Chinese Academy of Science for financial support through the Bairen project.
Notes and references
- (a) S. H. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS; (b) N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179 RSC; (c) S. H. Zhou, G. S. Jackson and B. Eichhorn, Adv. Funct. Mater., 2007, 17, 3099 CrossRef CAS; (d) I. C. Chiang and D. H. Chen, Adv. Funct. Mater., 2007, 17, 1311 CrossRef CAS; (e) Y. Sun, B. Wiley, Z. Y. Li and Y. Xia, J. Am. Chem. Soc., 2004, 126, 9399 CrossRef CAS; (f) N. C. King, R. A. Blackley, M. L. Wears, D. M. Newman, W. Z. Zhou and D. W. Bruce, Chem. Commun., 2006, 3414 RSC.
- (a) D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS; (b) R. W. J. Scott, O. M. Wilson, S. K. Oh, E. A. Kenik and R. M. Crooks, J. Am. Chem. Soc., 2004, 126, 15583 CrossRef CAS; (c) K. A. Guy, H. Xu, J. C. Yang, C. J. Werth and J. R. Shapley, J. Phys. Chem. C, 2009, 113, 8177 CrossRef CAS; (d) P. Dash, T. Bond, C. Fowler, W. Hou, N. Coombs and R. W. J. Scott, J. Phys. Chem. C, 2009, 113, 12719 CrossRef CAS.
- (a) R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845 CrossRef CAS; (b) S. H. Sun, Adv. Mater., 2006, 18, 393 CrossRef CAS.
- Y. Hou, H. Kondoh, T. Kogure and T. Ohta, Chem. Mater., 2004, 16, 5149 CrossRef CAS.
- (a) N. Toshima, M. Harada, Y. Yamazaki and K. Asakura, J. Phys. Chem., 1992, 96, 9927 CrossRef CAS; (b) N. Toshima and Y. Wang, Langmuir, 1994, 10, 4574 CrossRef CAS.
- N. Zheng, J. Fan and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 6550 CrossRef CAS.
- Y. W. Lee, N. H. Kim, K. Y. Lee, K. Kwon, M. Kim and S. W. Han, J. Phys. Chem. C, 2008, 112, 6717 CrossRef CAS.
- M.-L. Wu, D.-H. Chen and T.-C. Huang, Langmuir, 2001, 17, 3877 CAS.
- (a) N. Zettsu, J. M. McLellan, B. Wiley, Y. D. Yin, Z. Y. Li and Y. N. Xia, Angew. Chem., Int. Ed., 2006, 45, 1288 CrossRef CAS; (b) Y. Xiong, J. Chen, B. Wiley, Y. Xia, Y. Yin and Z. Y. Li, Nano Lett., 2005, 5, 1237 CrossRef CAS; (c) F. Bonet, V. Delmas, S. Grugeon, R. H. Urbina, P. Y. Silvert and K. Tekaia-Elhsissen, Nanostruct. Mater., 1999, 11, 1277 CrossRef CAS.
- (a) A. K. Sra, T. D. Ewers and R. E. Schaak, Chem. Mater., 2005, 17, 758 CrossRef CAS; (b) D. I. Garcia-Gutierrez, C. E. Gutierrez-Wing, L. Giovanetti, J. M. Ramallo-Lopez, F. G. Requejo and M. Jose-Yacaman, J. Phys. Chem. B, 2005, 109, 3813 CrossRef CAS; (c) R. Harpeness and A. Gedanken, Langmuir, 2004, 20, 3431 CrossRef CAS; (d) P. Lu, T. Teranishi, K. Asakura, M. Miyake and N. Toshima, J. Phys. Chem. B, 1999, 103, 9673 CrossRef CAS; (e) Y. Wang, H. Liu and N. Toshima, J. Phys. Chem., 1996, 100, 19533 CrossRef CAS; (f) S. Alayoglu and B. Eichhorn, J. Am. Chem. Soc., 2008, 130, 17479 CrossRef CAS.
- P. Mulvaney, Langmuir, 1996, 12, 788 CrossRef CAS.
- H. Kobayashi, M. Yamauchi, R. Ikeda and H. Kitagawa, Chem. Commun., 2009, 4806 RSC.
- J. P. Ge, W. Chen, L. P. Liu and Y. D. Li, Chem.–Eur. J., 2006, 12, 6552 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures and experimental conditions, a photograph of the color change for the PdAu nanoparticles in hexane solution, UV-vis spectra characterizations of PdAu, PdAg, PdCu, AuPt and AuAg nanoparticles, XRD and EDX of PdAg and PdCu nanoparticles, and TEM images of PdCu nanoparticles at low-magnification and superlattices of PdAg and AuAg nanoparticles. See DOI: 10.1039/b920169f |
| This journal is © The Royal Society of Chemistry 2010 |
