Simultaneous determination of pramoxine HCl and benzalkonium chloride in wound care solutions by HPLC
Panagiotis
Tavlarakis
ab,
Jonine
Greyling
a and
Nicholas H.
Snow
*b
aGlobal R&D Operations, Analytical Development, Johnson & Johnson Group of Consumer Companies, 185 Tabor Road, Morris Plains, NJ 07950
bDepartment of Chemistry and Biochemistry, Center for Academic Industry Partnership, Seton Hall University, 400 South Orange Avenue, South Orange, NJ 07079. E-mail: Nicholas.snow@shu.edu; Fax: +973-761-9772; Tel: +973-761-9035
First published on 31st March 2010
Abstract
A simple gradient reversed-phase high performance liquid chromatography (HPLC) method was developed for the simultaneous determination of total benzalkonium chloride (BAC) and pramoxine HCl in wound care solutions. Laboratory formulations were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with water and injected into a HPLC system equipped with a Phenomenex, Luna CN column (100 Å, 5μm, 250 mm × 4.6 mm) in order for BAC and pramoxine to separate from other excipients and be detected by a UV detector (λ = 262 nm). The mobile phase was 0.075 mM sodium acetate trihydrate buffer (pH = 5.0) using multi-ramp gradient elution with acetonitrile. Quantitation was achieved by direct comparison of the peaks of BAC and pramoxine HCl of the sample to a reference standard of known concentrations. A stress study with acid, base, peroxide, heat, and light indicated no interference from drug product or excipients. The mean recovery results for both pramoxine and benzalkonium chloride at 100% level were 100.5 ± 0.3%, 100.5 ± 0.1% respectively (mean ± SD, n = 6). In this report, the full experimental results from developing and validating this method are presented.
10 with water and injected into a HPLC system equipped with a Phenomenex, Luna CN column (100 Å, 5μm, 250 mm × 4.6 mm) in order for BAC and pramoxine to separate from other excipients and be detected by a UV detector (λ = 262 nm). The mobile phase was 0.075 mM sodium acetate trihydrate buffer (pH = 5.0) using multi-ramp gradient elution with acetonitrile. Quantitation was achieved by direct comparison of the peaks of BAC and pramoxine HCl of the sample to a reference standard of known concentrations. A stress study with acid, base, peroxide, heat, and light indicated no interference from drug product or excipients. The mean recovery results for both pramoxine and benzalkonium chloride at 100% level were 100.5 ± 0.3%, 100.5 ± 0.1% respectively (mean ± SD, n = 6). In this report, the full experimental results from developing and validating this method are presented.
1. Introduction
Benzalkonium chloride (BAC) is a mixture of alkylbenzyl dimethylammonium chlorides of various alkyl chain lengths with a general formula: [C6H5CH2N(CH3)2R]Cl, in which R represents a mixture of alkyls with n-C12H25, n-C14H29, n-C16H33 and n-C18H37 comprising the major portion of the BAC. The C12–C16 homologues are most common, so these were the focus of this study. The chemical structure of benzalkonium chloride is presented in Fig. 1. It is commonly used as an antiseptic with the greatest activity associated with the C12–C14 alkyl derivatives.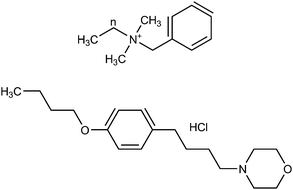 | ||
| Fig. 1 Chemical structures of benzalkonium chloride (n = length of alkyl chain) (top) and pramoxine hydrochloride (bottom). | ||
BAC has been in clinical use since 1935. It is an ingredient in a wide variety of prescription and over-the-counter products and is one of the typical preservative systems used in many topical applications.1,2 BAC is generally found in topical solutions for cleaning, minor wound care and disinfecting in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 dilution with water, or about 0.133%. For major wounds, mucous membrane and ophthalmic applications, concentrations are usually 10–50 times lower. Online searching of commercially available products shows many available with concentrations of this magnitude. The BAC homologues do not possess identical bactericidal activity. In general, C12 homologue is most effective against yeast and fungi, C14 homologue against gram-positive bacteria, and C16 homologue against gram-negative bacteria.3,4 Recently, benzalkonium chloride was shown to enhance antibacterial efficacy of antibiotics such as gatifloxacin.5 Many analytical methods (HPLC and HPCE) have been developed to determine BAC in a variety of products.6–12 These methods generally employ more extensive sample preparation or only quantify BAC without considering other components and none include the combination of BAC with pramoxine HCl.
750 dilution with water, or about 0.133%. For major wounds, mucous membrane and ophthalmic applications, concentrations are usually 10–50 times lower. Online searching of commercially available products shows many available with concentrations of this magnitude. The BAC homologues do not possess identical bactericidal activity. In general, C12 homologue is most effective against yeast and fungi, C14 homologue against gram-positive bacteria, and C16 homologue against gram-negative bacteria.3,4 Recently, benzalkonium chloride was shown to enhance antibacterial efficacy of antibiotics such as gatifloxacin.5 Many analytical methods (HPLC and HPCE) have been developed to determine BAC in a variety of products.6–12 These methods generally employ more extensive sample preparation or only quantify BAC without considering other components and none include the combination of BAC with pramoxine HCl.
Pramoxine HCl [C17H27NO3·HCl] is a local anaesthetic with a wide range of applicability in creams and lotions. It is used to block the pathway of pain along the nerve fibers, allowing pain relief where the medicine was applied. Pramoxine HCl is used as an anti itch and pain medication for a variety of conditions including skin irritations and rashes, hemorrhoids and minor wound care. In creams, ointments and solutions it is typically at 1% concentration. The chemical structure of Pramoxine HCl is presented in Fig. 1. In using pramoxine HCl, there is the potential for reaction with contaminants on the skin, leading to a diminished numbing effect.13 Decreased efficacy of topical anaesthetic creams containing pramoxine HCl was recently reported in the presence of benzoyl peroxide, which is the most widely used topical agent for acne.14 Recently pramoxine HCl was reported to be more effective than a control lotion in the treatment of uremic pruritus in adult hemodialysis patients.15 Pramoxine HCl is generally assayed by HPLC, but the most recent literature is approximately 25 years old.16,17
In this work we describe a new HPLC method for the simultaneous analysis of total benzalkonium chloride (the combined response of the homologues) and pramoxine HCl in wound care solutions. This represents an improvement over the separate USP methods for total BAC and pramoxine HCl, which involve a titration and an HPLC method, respectively.18 As pharmaceutical manufacturers explore new formulations and combinations of active pharmaceutical ingredients and excipients, there is a continuous challenge to adapt analytical methods to the new combinations of analytes and potential interferents. This new method is a stability indicating method and was successfully validated based on International Conference on Harmonization guidelines.19
2. Materials, equipment and method
2.1. Chemicals and solvents
The raw materials for pramoxine HCl (Abbott Laboratories, Abbott Park, Illinois, USA) and BAC (Century Pharmaceuticals, Indianapolis, USA) were provided by the Johnson and Johnson product development group. ACS reagent grade sodium acetate trihydrate and reagent grade glacial acetic acid were purchased from Sigma Aldrich (Saint Louis, Missouri, USA). HPLC grade acetonitrile was purchased from J.T. Baker (Phillipsburg, NJ, USA). Water was obtained using a Milli-Q (Millipore, Milford, MA) purification system installed in our laboratory.2.2. Equipment
An Alliance HPLC system (Waters Corporation, Milford, Massachusetts, USA) was used for the method development and method validation. The Alliance LC system was equipped with 2695 separation module, 2487 UV detector and 996 photodiode array detector. Data collection and processing was done using Empower (Waters Corporation, Milford, MA USA).2.3 Sample and standard preparation
Laboratory wound care formulations were prepared by combining BAC and pramoxine HCl with water at 0.133% and 1% concentrations by mass, respectively. There were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with water in a 100 mL volumetric flask prior to HPLC analysis. Calibration standards were prepared in a concentration range of 0.05–2.0 mg mL−1 of benzalkonium chloride and 0.40–1.60 mg mL−1 of pramoxine HCl, to generate 5-point calibration curves for each by dissolving the appropriate amounts of each in 70 mL of water in 100 mL volumetric flasks and diluting to the mark with additional water.
10 with water in a 100 mL volumetric flask prior to HPLC analysis. Calibration standards were prepared in a concentration range of 0.05–2.0 mg mL−1 of benzalkonium chloride and 0.40–1.60 mg mL−1 of pramoxine HCl, to generate 5-point calibration curves for each by dissolving the appropriate amounts of each in 70 mL of water in 100 mL volumetric flasks and diluting to the mark with additional water.
2.4. Method
Gradient reversed phase HPLC with UV detection was employed. The chromatographic column used was a Phenomenex, Luna CN, 100 Å, 5 μm, 250 mm × 4.6 mm. The flow was kept at 1.0 ml min−1 during the length of the run and the column temperature was 40 °C. The detector wavelength was 262 nm and the injection volume was 30 μl. Mobile phase A was 0.075 M sodium acetate trihydrate buffer with pH value adjusted to 5.0 with acetic acid. Mobile phase B was acetonitrile. Gradient elution was used, starting at 48% A and 52% B, holding for 11 min, then ramping to 100% B in 16 min (total run time = 27 min). The column was then returned to the initial condition and equilibrated for 8 min. Time between injections was 35 min. Total BAC was determined by summing the peak areas for each homologue and comparing this total to known standards. Pramoxine HCl was determined by comparing its peak area to known standards.2.5. Method validation
Method validation was performed in accordance to ICH guidelines and internal standard operating procedures for stability indicating methods. Both BAC and pramoxine HCl were considered to be active components for the purposes of this method. The test parameters are presented in the same order as they were investigated during the method validation.2.5.5.1. System precision. System precision was determined using six replicate measurements of a 100% theoretical standard solution containing benzalkonium chloride and pramoxine HCl. The error contributed by the system, independent of the sample preparation, should be less than the acceptance criteria of 2.0%.
2.5.5.2. Repeatability. The repeatability, which is the error contributed by sample preparation, was determined by six identical sample preparations of the same lot.
2.5.5.3. Intermediate Precision. In order to evaluate the degree of agreement among test results obtained from multiple samplings of the same lot of samples on a different day using a different instrument, column and analyst, with six identical sample preparations of the same lot.
2.5.6.1. Method parameters. Robustness of a method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters. The method parameters under test were wavelength (±4 nm), flow (±0.1 ml min−1) and pH (±0.2 pH units) of the buffer in the mobile phase. These parameters were changed one at a time. System suitability and sample runs were both conducted with unchanged method parameters and modified parameters.
2.5.6.2. Solution Stability. Standard and sample solutions were stored at room temperature and tested at initial, 72 h and 240 h of standing. The solutions were tested against a freshly prepared standard at each time point.
3. Results and discussion
3.1 Method development
There are many factors to consider when developing chromatographic methods. One of the factors is the choice of the proper detection scheme which depends on analyte properties. UV detection was selected since both benzalkonium chloride and pramoxine HCl have a UV chromophore. Detector wavelength selection (λ = 262 nm) was made based on the maximum absorbance of BAC because it was about ten times lower concentration compared to pramoxine HCl. Ideally for reversed phase HPLC methods several columns should be tested based on separation ability in the desired pH range. However, the first column tested, which is used in our laboratory for many analyses, proved capable of adequately separating all components and is a very common stationary phase and geometry, so testing of other columns was not necessary. In general, the Luna-CN column is used for separations of amines and acids and carbonyl-containing compounds, so it was an appropriate choice for this separation. Due to its multicomponent nature, BAC is difficult to analyze. The method should be quantitative and qualitative in order to identify and distinguish the homologue components from each other (system suitability criteria) and from pramoxine HCl.The concentration of pramoxine HCl in wound care solutions is generally about ten times higher than that of BAC. Preliminary studies indicated that for such a large difference in concentration, gradient elution is more suitable than isocratic elution, even though isocratic separations are often more reproducible and more predictable than gradient separations. Many attempts were made to elute all components isocratically but longer retention times and poor resolution between the BAC homologues were observed and made the method impractical. Since benzalkonium chloride has been known for its adsorption in membrane material, sample solutions were not filtered during sample preparation.21
System suitability parameters were selected to provide confidence that the method is capable of determining BAC and pramoxine HCl in wound care solutions:injection precision for both active components, resolution between C12, C14 and C14, C16 homologues, and tailing factor of BAC homologues (C12, C14, C16). In addition, check standard conformity and bracketing standard conformity were proved during each chromatographic run. All of these tests produced results within the guidelines of our standard operating procedures for system suitability. Typical chromatograms for pramoxine HCl and benzalkonium chloride system suitability standard solutions are shown in Fig. 2. Fig. 2A is a full scale chromatogram showing the pramoxine HCl peak to full scale while Fig. 2B is an expanded chromatogram showing the BAC homologue peaks full scale. Examination of both the full scale and expanded chromatograms shows satisfactory peak shape and separation of all four analytes. Resolution of all components from each other and from the small additional peaks is sufficient that the separation should be rugged and robust.
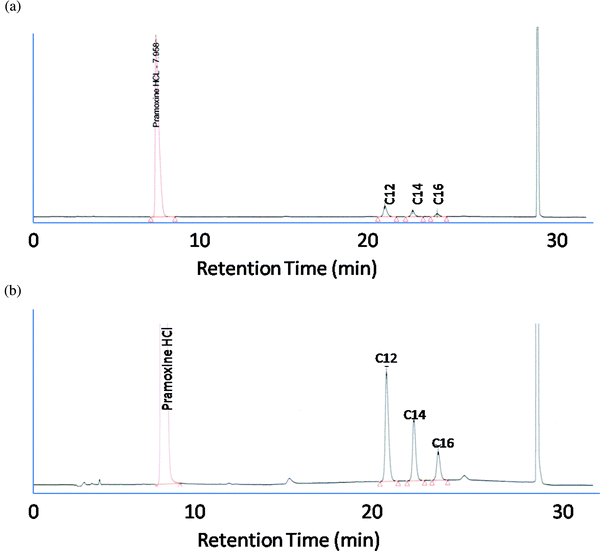 | ||
| Fig. 2 System suitability chromatogram (a): full scale chromatogram, (b): expanded chromatogram. | ||
3.2 Validation
The specificity of the method was tested and no interference from impurities or degradants was observed for the BAC and pramoxine HCl peaks from placebo and forced degradation samples using acid, base, light peroxide and heating. To ensure no extraneous peak co-eluted with the BAC homologues or pramoxine HCl, a diode array detector was used to check peak purity using the full UV spectrum at several points on each peak. Fig. 3 shows overlay chromatograms at 262 nm showing no interference between pramoxine hydrochloride, three benzalkonium chloride homologues, excipients and impurities. Three chromatograms are shown on each plot: a placebo prepared including most components in a commercial formulation, a commercial sample and a standard. The extra peaks in the placebo and commercial formulation are other components present in those samples and not in the standard. Both full scale and expanded views are shown to demonstrate the satisfactory separation and peak shape of components at both the higher concentrations indicative of pramoxine HCl and the lower levels of total BAC.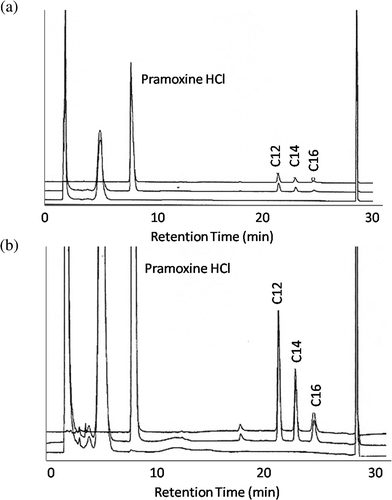 | ||
| Fig. 3 Overlay chromatogram of placebo (bottom), sample (middle) and standard (top). (a): Full scale chromatogram (b): expanded chromatogram. | ||
In each of the situations where standards, commercial samples and placebos were stressed, there were a few situations where additional peaks or baseline drift appeared on some chromatograms. None of these situations showed any interference with the analyte responses, chromatographic performance or quantitation.
Linearity of the method was established. Five standard concentrations of BAC and pramoxine were prepared and injected. A linearity graph was prepared by plotting the concentration versus the response of the actives. The solutions covered a range of 40–160% of the laboratory formulation concentration. The method was linear in this range with R2 values of 0.9999 and no apparent deviation of data points from the best fit lines. No carryover was observed into blank injections immediately after the highest level linearity standard of both actives, ensuring independence of the samples. This calibration range allows the analysis of a variety of possible formulation combinations using BAC and Pramoxine HCl. Similar linearity was observed when standards were prepared and carried through the simple dilution process used for analytical samples.
3.3 Precision and accuracy
System precision was established by six replicate measurements of a laboratory formulation containing both components. The %RSD for total BAC and pramoxine HCl were found to be 0.1% and 1.5% respectively. The repeatability of the method was evaluated by six identical sample preparations of a homogeneous batch of a formulated product and the results were found to be within a generally accepted specification range of ±2%. The percent relative standard deviation of the six preparations for both active components was found to be 0.7%.In order to determine method precision and validate the results, the experiment was repeated at a different work site, using different instruments and different columns. The experimental mean agreement using the laboratory formulation between the two sites were found to be 2.1% and 0.3% of label claim for total BAC and pramoxine HCl respectively, both of which are within our acceptance criteria. Criteria for the analysis are: %RSD ≤2.0% and agreement of the means should be ±3.0%. Low BAC/high pramoxine or high BAC/low pramoxine were not tested. If the high/high accuracy results had failed, there would have been a need to test these other cases. Detailed results are presented in Table 1.
| Replicate | % Label Claim (%LC) | |||
|---|---|---|---|---|
| Benzalkonium Chloride | Pramoxine HCl | |||
| Lab 1 | Lab 2 | Lab 1 | Lab 2 | |
| 1 | 98.9 | 100.6 | 100.7 | 100.3 |
| 2 | 99.0 | 100.8 | 101.1 | 100.1 |
| 3 | 98.5 | 101.3 | 101.0 | 100.5 |
| 4 | 98.5 | 100.0 | 100.8 | 100.0 |
| 5 | 97.2 | 100.5 | 99.3 | 100.4 |
| 6 | 99.1 | 100.4 | 101.0 | 100.4 |
| Mean | 98.5 | 100.6 | 100.6 | 100.3 |
| %RSD | 0.7 | 0.4 | 0.7 | 0.2 |
| Mean Agreement | 2.1 | 0.3 | ||
Accuracy was established by assaying three different concentration levels 70%, 100%, and 130% of the laboratory formulation concentration for both active components. Six preparations of placebo, at each level, were spiked with standards of both BAC and pramoxine HCl and injected into the HPLC system. Results are reported in Table 2 with total BAC mean recovery values varying from 100.4 to 101.0% label claim. The pramoxine HCl mean recovery values varied from 100.2% to 100.9% label claim. No bias was observed for the two active components, since the results for the mean recovery from all three accuracy levels were not significantly lower or higher (±1.5%) than the theoretical value.
| Prep # | BAC | Pramoxine HCl | ||||
|---|---|---|---|---|---|---|
| Mean Recovery Value %LC | Mean Recovery Value %LC | |||||
| 70% Level 0.9 mg mL−1 | 100% Level 1.3 mg mL−1 | 130% Level 1.8 mg mL−1 | 70% Level 7 mg mL−1 | 100% Level 10 mg mL−1 | 130% Level 13 mg mL−1 | |
| 1 | 100.2 | 100.4 | 101.2 | 100.6 | 100.9 | 100.5 |
| 2 | 100.4 | 100.4 | 100.9 | 100.8 | 100.6 | 100.3 |
| 3 | 100.6 | 100.5 | 101.0 | 101.1 | 100.5 | 100.1 |
| 4 | 100.4 | 100.4 | 100.8 | 100.9 | 100.4 | 100.1 |
| 5 | 100.4 | 100.5 | 100.9 | 101.1 | 100.3 | 100.2 |
| 6 | 100.2 | 100.5 | 100.9 | 100.9 | 100.0 | 100.3 |
| Mean | 100.4 | 100.5 | 101.0 | 100.9 | 100.5 | 100.2 |
| %RSD | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 |
3.4 Ruggedness, robustness and stability
The method was unaffected by small, deliberate variations in chromatographic parameters and mobile phase preparation. The parameters tested were wavelength, flow and pH of the buffer. The robustness results have shown that the retention time and the peak shape were not affected by the parameters under testing. The variation that was observed between the normal method conditions and the changed parameters was from 0 to 0.3% for BAC and 0 to 0.4% for pramoxine HCl. The stability of the standard and sample solutions for both actives was evaluated. The results are displayed in Table 3 and show no significant decrease in response over a period of 240 h for both BAC and pramoxine HCl. None of the deliberate variations caused any loss of chromatographic resolution or increase in tailing factor.| Time Point | Benzalkonium Chloride Standard | Pramoxine HCI Standard | ||
|---|---|---|---|---|
| Response | % Difference from Initial | Response | % Difference from Initial | |
| Initial | 100.0 | — | 100.0 | — |
| 72 Hours | 99.5 | 0.5 | 100.1 | 0.1 |
| 240 Hours | 99.7 | 0.3 | 100.1 | 0.1 |
| Time Point | Benzalkonium Chloride Sample | Pramoxine HCI Sample | ||
|---|---|---|---|---|
| Response | % Difference from Initial | Response | % Difference from Initial | |
| Initial | 99.0 | — | 101.1 | — |
| 72 Hours | 99.2 | 0.2 | 99.8 | 1.3 |
| 240 Hours | 99.7 | 0.3 | 99.8 | 1.3 |
4. Conclusions
In this work, a new method for the simultaneous determination of total benzalkonium chloride and pramoxine HCl in wound care solutions is described. Simple dilution followed by gradient HPLC with UV-detection is employed, generating a very straightforward method that is currently in use for a variety of applications. The method fully separates the BAC homologues from pramoxine HCl and other excipients. Validation for this method was performed according to ICH guidelines and met all acceptance criteria. The method is precise, with relative standard deviation about 1%, accurate, with recoveries from spiked samples between 100 and 101% and linear at concentration ranges, 4–16 mg mL−1 for pramoxine HCl and 0.5–2 mg mL−1 for total BAC, encompassing our laboratory formulations and the known concentrations of many products containing these substances.5. Acknowledgments
The authors are grateful for the support of Johnson and Johnson, Group of Consumer Companies, especially the product development group which provided the raw materials used in these studies. We would also like to acknowledge the technical assistance and advice from Ken Holeva who provided samples, standards and formulation background. Nicholas Snow gratefully acknowledges the Sanofi-Aventis Foundation which provided financial support to the Center for Academic Industry Partnership at Seton Hall University.6. References
- L. P. Labranche, S. N. Dumont, S. Levesque and A. Carrier, J. Pharm. Biomed. Anal., 2007, 43, 989–993 CrossRef CAS.
- G. Parhizkari, G. Delker, R. B. Miller and C. Chen, Chromatographia, 1995, 40, 155–158 CAS.
- E. G. Romanowski, F. S. Mah, R. P. Kowalski, K. A. Yates and Y. J. Gordon, J. Ocul. Pharmacol. Ther., 2008, 24(4), 380–384 CrossRef CAS.
- H. Aki and Y. Kawasaki, Thermochim. Acta, 2004, 416, 113–119 CrossRef CAS.
- S. J. Prince, H. J. McLaury, L. V. Allen and P. McLaury, J. Pharm. Biomed. Anal., 1999, 19, 877–882 CrossRef CAS.
- J. E. Parkin, J. Chromatogr., A, 1993, 635, 75–80 CrossRef CAS.
- J. Dudkiewicz-Wilczynska, J. Tautt and I. Roman, J. Pharm. Biomed. Anal., 2004, 34, 909–920 CrossRef CAS.
- György Ambrus, Lloyd T. Takahashi and Patricia A. Marty, J. Pharm. Sci., 1987, 76, 174–176 CrossRef CAS.
- A. Gomez-Gomar, M. M. Gonzalez-Aubert, J. Garces-Torrents and J. Costa-Segarra, J. Pharm. Biomed. Anal., 1990, 8, 871–876 CrossRef CAS.
- G. Santoni, A. Tonsini, P. Gratteri, P. Mura, S. Furlanetto and S. Pinzauti, Int. J. Pharm., 1993, 93, 239–243 CrossRef CAS.
- Tony Y. Fan and G. Michael Wall, J. Pharm. Sci., 1993, 82, 1172–1174 CAS.
- P. Rojsitthisak, W. Wichitnithad, O. Pipitharome, K. Sanphanya, P. Thanawattanawanich, J. Pharm. Sci. Technol, 2005, 59, 332–337 Search PubMed.
- J. J. Merianos, in: S. S. Block (Ed.), Disinfection, Sterilization, and Preservation, Lea and Febiger, PA, 1991, p. 225 Search PubMed.
- C. G. Burkhart and C. N. Burkhart, Dermatologic Surgery, 2005, 31, 1479–1480 CAS.
- T. A. Young, T. S. Patel, F. Camacho, A. Clark, B. I. Freedman, M. Kaur, J. Fountain, L. L. Williams, G. Yosipovitch and A. B. Fleischer Jr, J. Dermatol. Treat., 2009, 20(2), 76–81 CrossRef CAS.
- R. Weinberger, B. Mann and J. Posluszny, J. Pharm. Sci., 1979, 69, 475–477.
- R. Gill, R. W. Abbott and A. C. J. Moffat, J. Chromatogr., A, 1984, 301, 155–63 CrossRef CAS.
- United States Pharmacopeia 32 National Formulary 27 Monographs: Benzalkonium Chloride and Pramoxine HCl, United States Pharmacopeia, 2010 Search PubMed.
- ICH Topic Q 2 B Validation of Analytical Procedures: Methodology (Having reached step 4 of the ICH process at the ICH Steering Committee meeting on 6 November 1996, and incorporated in the core guideline in November 2005, this guideline is recommended for adoption to the three regulatory parties to ICH, 6–13.).
- United States Pharmacopeia 31/National Formulary 26, General Chapter <621> Chromatography, 2008.
- T. Bin, A. K. Kulshreshtha, R. Al-Shakhshir and S. L. Hem, Pharm. Dev. Technol., 1999, 4(2), 151–165 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
