Manganese octahedral molecular sieves catalyzed tandem process for synthesis of quinoxalines
Shanthakumar
Sithambaram
a,
Yunshuang
Ding
b,
Weina
Li
b,
Xiongfei
Shen
b,
Faith
Gaenzler
a and
Steven L.
Suib
ab
aDepartment of Chemistry, University of Connecticut, U-3060, 55 North Eagleville Rd., Storrs, Connecticut, 06269-3060, USA. E-mail: Steven.Suib@uconn.edu; Fax: (860) 486-2981; Tel: (860) 486-2797
bDepartment of Chemical, Materials, and Biomolecular Engineering, University of Connecticut, U-3060, 55 North Eagleville Rd., Storrs, Connecticut, 06269-3060, USA
First published on 12th September 2008
Abstract
An efficient environmentally benign tandem synthetic route to prepare quinoxalines leading to 100% yields using reusable manganese oxide octahedral molecular sieves (OMS-2) is described.
Single-pot tandem reactions involving catalysis have recently become an important methodology in chemistry.1 Multi-step organic syntheses are common in the fine chemical industry, and they suffer from several disadvantages. Such reactions are often carried out non-catalytically using relatively large amounts of reagents that produce many kilograms of waste per kilogram of final product. In addition, the separation and purification steps needed after each conversion step produce waste heat. Going from traditional step-by-step methods to a one-pot coupled conversion saves raw materials and energy and reduces waste.2
Quinoxalines are a versatile class of nitrogen containing heterocyclic compounds and they constitute useful intermediates in organic synthesis.3 Quinoxalines have been reported to be biocides,4pharmaceuticals,5 and organic semiconductors.6 Conventionally, quinoxalines are synthesized by a double condensation reaction involving a dicarbonyl and ortho-phenylenediamine.7 Due to the highly reactive nature of the dicarbonyls, alternative routes have been proposed recently. Antoniotti and Donach have reported one of these methods to synthesize quinoxalines from epoxides and ene-1,2-diamines.8 Active manganese oxide and molecular sieves in combination or manganese oxides in combination with microwaves have also been used in producing quinoxalines.9 These processes, however, require excessive amounts of manganese oxide as stoichiometric oxidants and scaling them up for industrial processes can lead to the formation of large amounts of toxic waste leading to environmental issues. In additional studies, Robinson and Taylor reported a homogeneous catalytic process utilizing Pd(OAc)2, RuCl2(PPh3)2 to synthesize quinoxalines from hydroxy ketones,10 and recently a copper catalyzed oxidative cyclization process has been reported.11 An improved ruthenium catalyzed direct approach to synthesize quinoxalines from diols and ortho-diamines has also been reported.12 These processes are efficient, but suffer from the major drawback that the catalysts cannot be recovered and reused.
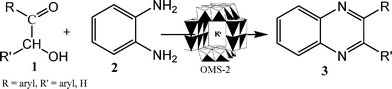
In this communication we report a highly efficient catalytic single pot tandem synthetic route (Scheme 1) to form quinoxalines (3) from hydroxy ketones (1) and diamines (2) using manganese oxide octahedral molecular sieves (K-OMS-2). OMS-2 materials have been used as catalysts for the oxidation and condensation reactions in our laboratory.13K-OMS-2 is a cryptomelane-type manganese oxide with the composition KMn8O16·nH2O and consists of MnO6 octahedral units, which are edge and corner shared to form a 2 × 2 tunnel structure.14 This alternative process to synthesize quinoxalines with K-OMS-2 does not require any additives or promoters as in the processes described above for this transformation and the reaction times are shorter. Moreover, the K-OMS-2 catalysts are relatively inexpensive, easy to prepare and can be reused many times without loss of activity. The reaction proceeds via two steps, i.e. the oxidation of the hydroxy ketones to their diketones and then the condensation of the diketones with a diamine to form the final product, quinoxaline.
 | ||
| Scheme 1 | ||
The synthesis of a quinoxaline with α-pyridoin and 1,2-phenylenediamine was attempted with K-OMS-2 catalysts prepared by different methods (Table 1). K-OMS-2 prepared by solvent free (SF) methods is known as K-OMS-2SF, K-OMS-2 prepared by reflux (R) methods is known as K-OMS-2R and K-OMS-2 synthesized by high temperature (HT) methods is known as K-OMS-2HT. The main criterion for selecting these catalysts was based on their surface areas. The K-OMS-2SF prepared by the solvent-free method had the highest surface area, while K-OMS-2HT prepared by a high temperature method had the lowest surface area. Interestingly, all the catalysts produced quinoxaline in high to moderate yields. The use of K-OMS-2R resulted in a 98% quinoxaline yield, while the use of K-OMS-2SF and K-OMS-2HT gave 74% and 49% yield respectively. In all cases, the selectivity for quinoxaline was 100% and no other side products were formed. The difference in the yields of quinoxalines can be related to their intrinsic properties. The surface area and the average oxidation number of the catalysts in combination seem to play a key role in the process. The surface area is related to crystallite size of the catalysts. The average oxidation number that represent the ratio of Mn2+, Mn3+, and Mn4+ which influences the strength of Lewis acidity of the catalysts may also play a role. However, a detailed investigation to relate the catalytic activity to their properties is still in progress.
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst c | AOSd | Surface area/m2 g−1 | crystallite size/nm | Yield (%)e | TOF f/h−1 |
| a 1 mmol α-pyridoin, 2 mmol 1,2-phenylenediamine and 50.0 mg catalyst stirred in 10 mL toluene under reflux for 1 h. b 50 mg = 0.0625 mmol K-OMS-2. c Catalyst preparation methods, SF—solvent free, R—reflux, HT—high temperature. d Average oxidation number determined by potentiometric titrations (ref. 15). e Determined by GC-MS and NMR (yield = conversion × selectivity). f Turnover frequency = moles of converted substrate/(moles of catalyst × reaction time in h). | ||||||
| 1 | K-OMS-2SF | 3.72 | 156 | 10 | 74 | 11.8 |
| 2 | K-OMS-2R | 3.90 | 90 | 18 | 98 | 15.7 |
| 3 | K-OMS-2HT | 3.85 | 13 | 20 | 49 | 7.8 |
This protocol was then extended to a range of substrates using K-OMS-2 as catalysts prepared by reflux methods and the results are listed in Table 2. Quinoxaline synthesis with 2-hydroxyacetophenone (1a) and 1,2-phenylenediamine (2a) gave 100% yield to the corresponding quinoxaline (3a). Benzoin (1b) gave a moderate yield to its quinoxaline (3b), while heterocyclic substrates pyridoin (1e) and furoin (1d) showed good yields to their corresponding quinoxalines, 98% (3e) and 84% (3d) respectively. Hetero-atoms in the cyclic structures also seem to play a role in the formation of the quinoxalines. Anisoin (1c) gave the lowest yield of all the hydroxy ketone substrates tried. The presence of the electron donating OCH3group in the benzene ring may retard the nucleophilic attack on the in situ formed dicarbonyl leading to a lower yield. The effects of electron withdrawing and electron donating substituents in the nucleophile diamine substrate were also studied. The reaction between furoin (1d) and 4-methoxy-1,2-phenylenediamine (2c) gave an 89% yield for 3g, indicating that the presence of an electron donating methoxy group in the diamine enhances its nucleophilicity. On the other hand, 4-nitrophenylenediamine (2b) with furoin (1d) yielded only 20% quinoxaline 3f. The other product obtained was the intermediate diketone, furil in 22% yield. These results show that electron withdrawing nitro groups in the diamine retard its nucleophilicity. The use of chloro-substituted diamines (2d) led to enhanced yields for their quinoxalines (entries 8–12).
Alternative solvent systems such as acetonitrile, THF, toluene, and benzene were evaluated in the formation of quinoxalines at their reflux temperatures. However, toluene gave the highest conversion among the solvents tried. The main reason for this could be that the highest reflux temperature attained with toluene aids in the oxidation step of quinoxaline. In consideration of its abundance, economic, and environmental attractiveness, water was also tried as a solvent for the reaction. The reaction in water afforded only a 9% yield. Additional studies were performed to test the reusability of the catalyst. The reaction with α-pyridoin and 1,2-phenylenediamine was carried out over four cycles with the same catalyst which was regenerated after each use by simply washing with acetone/methanol and water and heating to 250 °C. Due to the loss of catalysts during the filtration process after each reaction, a reduced amount of catalysts was used in the subsequent cycles. The yields of the “spent” catalysts used in all the cycles were comparable to yields for “fresh” catalysts (Table 3). The X-ray diffraction patterns of the regenerated catalysts indicated that the structure of K-OMS-2 was not altered during the reaction (Fig. 1). In order to prove that the reaction is heterogeneous, a standard leaching experiment was conducted. The catalyst was filtered at the reaction temperature, and the reaction was allowed to proceed without the catalyst. There was no change in yield observed even after 8 h indicating that no homogeneous catalysis was involved. Turnover frequency (TOF) for this catalytic process which is defined as moles of converted substrate per mole of catalyst per hour has been listed in Table 1. TOF of 15 have been achieved for this reaction with K-OMS-2 as catalyst and this value is very high compared to the similar process which requires 10–15 equivalents of active MnO2 in 20 h (TOF = 1.4 × 10−3).16
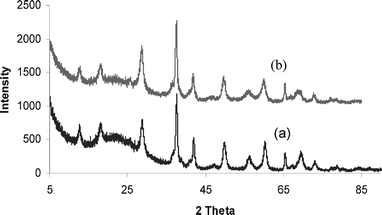 | ||
| Fig. 1 XRD patterns of K-OMS-2 catalyst in synthesis of quinoxalines: (a) “fresh” catalyst before reaction, (b) “spent” catalyst after 2nd cycle. | ||
Finally, the quinoxaline synthesis was carried out on a gram scale. One gram of pyridoin was reacted with one gram of 1,2-phenylenediamine under the same reaction conditions. The reaction occurred to produce the corresponding quinoxaline in 81% yield in one hour. This promising result suggests that this catalytic protocol can be extended to larger scale, milligram to gram scale reactions.
In summary, manganese octahedral molecular sieves efficiently catalyze the single-pot synthesis of quinoxalines from hydroxyl ketones and diamines. The reactions require only a catalytic amount of K-OMS-2 and do not require any additives or promoters for the reaction. The K-OMS-2 catalysts are environmentally benign and after a simple regeneration process can be reused without loss of activity.
Experimental section
The catalysts, K-OMS-2R by a reflux method17 and K-OMS-2SF by a solvent-free method18 were prepared according to literature procedures. The high temperature K-OMS-2HT19 was prepared by a combination of sol–gel and combustion methods with the Mn source as Mn(NO3)2. KNO3 and Mn(NO3)2 in a molar ratio of 1 : 5 were dissolved in distilled deionized water (solution A). Glycerol and KNO3 were mixed in a 1 : 10 ratio (solution B). Solutions A and B were mixed in deionized water with vigorous stirring to form a clear solution and then heated to 120 °C to form a gel (usually 5 h). The gel was then heated to 250 °C for 2 h to complete the combustion reaction. The black powder was then calcined at 600 °C for 3 h to obtain the final product.The reactions were carried out in batch reactors. A typical reaction procedure as follows: to a round-bottomed flask (50 mL), furoin (1 mmol, 192 mg), toluene (10 mL) as solvent, 4-methoxy-o-phenylenediamine (2 mmol, 276 mg), and K-OMS-2 catalyst (50.0 mg) were added. The mixture was stirred under reflux for 1 h at 110 °C in air. After the reaction time, the mixture was cooled; the catalyst was removed by filtration. A gas chromatography-mass spectroscopy (GC-MS) method was used for the identification and quantification of the product mixtures. GC-MS analyses were done using an HP 5890 series II chromatograph with a thermal conductivity detector coupled with an HP 5970 mass selective detector. An HP-1 column (non-polar cross linked siloxane) with dimensions of 12.5 m × 0.2 mm × 0.33 μm was used in the gas chromatograph. The products were also confirmed by 1H and 13C NMR data collected on a Brucker DRX-400 (400.144 MHz 1H, 100.65 MHz 13C). Silica-gel column chromatography after concentration afforded 2,3-bis(2-furyl)-6-methoxy quinoxaline (Table 2, Entry 7). m/z = 292, 1H NMR (400 MHz, CDCl3): δ = 3.83 (s, 3H), 6.43 (m, 3H), 6.55 (d, 1H, J = 4 Hz), 7.26 (dd, 1H, J = 4, 8 Hz), 7.51 (m, 2H), 7.87 (d, 1H, J = 8 Hz); 13C NMR (400 MHz, CDCl3): δ = 55.9, 106.5, 111.9, 112.0, 113.1, 123.8, 130.1, 136.7, 140.2, 142.4, 142.7, 143.7, 144.3, 149.5, 150.9, 151.1, 161.4.
Acknowledgements
The authors would like to thank the Chemical, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U. S. Department of Energy. The authors would also like to thank Dr Frank Galasso and Dr James Bobbitt for many helpful discussions.Notes and references
- (a) J. C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001–1020 CrossRef CAS; (b) J. L. Notre, D. V. Mele and C. G. Frost, Adv. Synth. Catal., 2007, 349, 432–440 CrossRef CAS; (c) A. Ajamian and J. L. Gleason, Angew. Chem., Int. Ed., 2004, 43, 3754–3760 CrossRef CAS.
- (a) T. L. Ho, Tandem Organic Reactions, Wiley, New York, 1992 Search PubMed; (b) R. Shoevaart and T. Kieboon, Chem. Innov., 2001, 31, 33–39.
- D. J. Brown, The Chemistry of Heterocyclic Compounds, Vol 61, Quinoxalines: Supplement II, Wiley, New York, 2004 Search PubMed.
- R. Sarges, H. R. Howard, R. G. Browne, L. A. Lebel and P. A. Seymour, J. Med. Chem., 1990, 33, 2240–2254 CrossRef CAS.
- L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5605–5606.
- S. Dailey, J. W. Feast, R. J. Peace, I. C. Sage, S. Till and E. L. Wood, J. Mater. Chem., 2001, 11, 2238–2243 RSC.
- (a) Z. Zhao, D. D. Wisnoski, S. E. Wolkenberg, W. H. Leister, Y. Wang and C. W. Lindsley, Tetrahedron Lett., 2004, 45, 4873–4876 CrossRef; (b) S. V. More, M. N. V. Sastry and C. -F. Yao, Green Chem., 2006, 8, 91–95 RSC.
- S. Antoniotti and E. Donach, Tetrahedron Lett., 2002, 43, 3971–3973 CrossRef.
- (a) S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Chem. Commun., 2003, 2286–2287 RSC; (b) S. Y. Kim, K. H. Park and Y. K. Chung, Chem. Commun., 2005, 1321–1323 RSC.
- R. S. Robinson and R. J. K. Taylor, Synlett., 2005, 6, 1003–1005.
- C. S. Cho and S. G. Oh, J. Mol. Catal. A: Chem., 2007, 276, 205–210 CrossRef CAS.
- C. K. Cho and S. G. Oh, Tetrahedran Lett., 2006, 47, 5633–5636 CrossRef CAS.
- (a) Y. C. Son, V. D. Makwana, A. R. Howell and S. L. Suib, Angew. Chem., Int. Ed., 2001, 40, 4280–4283 CrossRef CAS; (b) R. Kumar, L. J. Garces, Y. -C. Son, S. L. Suib and R. E. Malz, J. Catal., 2005, 236, 387–391 CrossRef CAS; (c) S. Sithambaram, R. Kumar, Y. C. Son and S. L. Suib, J. Catal., 2008, 253, 269–277 CrossRef CAS.
- (a) Y. F. Shen, R. P. Zerger, R. N. DeGuzman, S. L. Suib, L. McCurdy, D. I. Potter and C. L. O'Young, Science, 1993, 260, 511–515 CAS; (b) R. N. DeGuzman, Y. F. Shen, E. J. Neth, S. L. Suib, C. L. O'Young, S. Levine and J. M. Newsam, Chem. Mater., 1994, 6, 815–821 CrossRef CAS; (c) S. L. Suib, Curr. Opin. Solid State Mater. Sci., 1988, 3, 63–70; (d) S. L. Brock, N. Duan, Z. R. Tian, O. Giraldo, H. Zhou and S. L. Suib, Chem. Mater., 1998, 10, 2619–2628 CrossRef CAS.
- G. -G. Xia, W. Tong, E. N. Tolentino, N. -G. Duan, S. L. Brock, J. -Y. Wang, S. L. Suib and T. Ressler, Chem. Mater., 2001, 13, 1585–1592 CrossRef CAS.
- S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Org. Biomol. Chem., 2004, 2, 788–796 RSC.
- R. N. DeGuzman, Y -F. Shen, E. J. Neth, S. L. Suib, C. L. O'Young, S. Levine and J. M. Newman, Chem. Mater., 1994, 6, 815–821 CrossRef CAS.
- Y. -S. Ding, X. F. Shen, S. Sithambaram, S. Gomez, R. Kumar, M. B. Vincent and S. L. Suib, Chem. Mater., 2005, 17, 5382–5389 CrossRef CAS.
- X. F. Shen, PhD Thesis, University of Connecticut, 2007.
| This journal is © The Royal Society of Chemistry 2008 |