Clean coal conversion processes – progress and challenges
Fanxing Li and Liang-Shih Fan*
Department of Chemical and Biomolecular Engineering, The Ohio State University, 140 West 19th Avenue, Columbus, Ohio 43210, USA. E-mail: Fan.1@osu.edu; Fax: +001-614-292-3769; Tel: +001-614-688-3262
First published on 30th July 2008
Abstract
Although the processing of coal is an ancient problem and has been practiced for centuries, the constraints posed on today's coal conversion processes are unprecedented, and utmost innovations are required for finding the solution to the problem.
With a strong demand for an affordable energy supply which is compounded by the urgent need for a CO2 emission control, the clean and efficient utilization of coal presents both a challenge and an opportunity to the current global R&D efforts in this area. This paper provides a historical perspective on the utilization of coal as an energy source as well as describing the progress and challenges and the future prospect of clean coal conversion processes. It provides background on the historical utilization of coal as an energy source, along with particular emphasis on the constraints in current coal conversion technologies. It addresses the energy conversion efficiencies for current coal combustion and gasification processes and for the membrane and looping based novel processes which are currently under development at various stages of testing. The control technologies for pollutants including CO2 in flue gas or syngas are also discussed. The coal conversion process efficiencies under a CO2 constrained environment are illustrated based on data and ASPEN Plus® simulations. The challenges for future R&D efforts in novel coal conversion process development are also presented.
 F. Li | Fanxing Li received his B.S. degree in 2001 and his M.S. degree in 2004 in Chemical Engineering from Tsinghua University. He is a graduate research associate in the Department of Chemical and Biomolecular Engineering at The Ohio State University. He is currently working on a number of projects with Professor L. S. Fan including energy and environmental reaction engineering and clean coal conversion processes. |
 L. S. Fan | L. S. Fan is Distinguished University Professor and C. John Easton Professor in Engineering in the Department of Chemical and Biomolecular Engineering at The Ohio State University. His expertise is in fluidization and multiphase flow, powder technology and energy and environmental reaction engineering. He is a member of the U. S. National Academy of Engineering, and an Academician of the Academia Sinica. |
1. Background
Energy and global warming are two intertwined issues of significant magnitude in the modern era. With oil prices rising above $120/barrel and atmospheric CO2 levels increasing at a rate greater than 1.5 ppm each year,1–3 an urgent need exists for development of clean and cost effective energy conversion processes.Renewable energy sources such as hydro, wind, solar, geothermal, and biomass will help reduce anthropogenic CO2 emissions by mitigating fossil fuel consumption. However, with the high cost, geological constraints, and intermittency issues, renewable energy is not likely to contribute to a significant share of the total energy demands in the foreseeable future.4,5 Similarly, concerns over plant safety and radioactive waste disposal will impede the wide utilization of nuclear power.6 Thus, despite high crude oil and natural gas prices, fossil fuels will continue to provide more than 85% of the overall world energy consumption for the next several decades.7 US DOE studies indicate that the consumption of coal as an energy resource is more responsive to crude oil price fluctuations than renewable energy sources in the near term, and coal could regain its role as a major energy source by 2030.7Fig. 1 shows the impact of oil prices on the consumption of coal and other energy sources. The attractiveness of coal lies in its abundant reserves and stable prices when compared to both oil and natural gas.
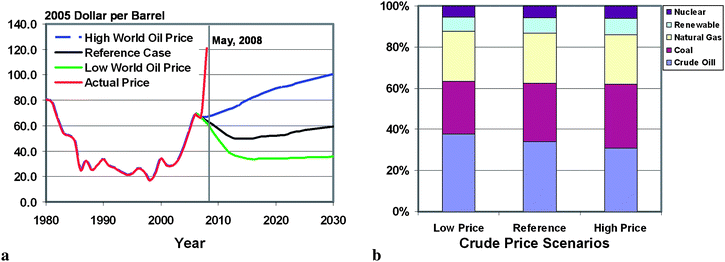 | ||
| Fig. 1 (a) Three different (long term) world oil price scenarios predicted by EIA; (b) world energy consumption in 2030 based on energy sources.7 | ||
Without the implementation of pollution control, enhanced coal usage will result in serious environmental impacts since coal contains various contaminants and is the most carbon-intensive energy source. Of major global concern is the fact that the combustion of fossil fuels releases 27 gigatons of CO2 each year.7,8 With increasing coal consumption, the anthropogenic CO2 emission rate may reach well over 40 gigatons per year within the next two decades in the absence of effective CO2 mitigation techniques.7,8 Therefore, modern coal conversion technologies need to be able to efficiently convert coal into useful products while controlling the CO2 emission. Unlike crude oil, which is primarily used as transportation fuels, coal is primarily used as a stationary source for electricity generation. Thus, CO2 capture from coal can be more readily implemented.
This article addresses clean coal conversion technologies from the process viewpoint. Coal combustion processes are first discussed along with the various options for pollutant control and CO2 capture. It is then followed by an overview of coal gasification processes. Advanced membrane and chemical looping based systems using gaseous feedstock as well as advanced direct coal chemical looping systems are illustrated. These advanced technologies that yield high energy conversion efficiencies are at various stages of development and are potentially deployable in the near or intermediate term.
2. Coal combustion processes
Archeological evidence indicates that humans have been burning coal for at least 4000 years.9–11 Throughout history, coal has been used to generate heat and to smelt metals. However, it was not until the 18th century that coal started to play an indispensible role in the economy. As an important fuel that propelled the industrial revolution,12,13 coal has been widely used since the 1700s to drive steam engines, in the operation of blast furnaces for metal production, in the production of cement, and in the generation of town gas for lighting and cooking. Since the late 19th century, coal has been used to power utility boilers for electricity generation.14 Although its dominance as an energy source was replaced by crude oil in the 1950s, coal is still the single most important fuel for electricity generation today, accounting for 40% of the electricity generated worldwide.7 The dominance of coal in electricity generation is expected to continue well into the 21st century.Presently, pulverized coal (PC) fired power plants account for more than 90% of the electricity generated from coal.15 The schematic flow diagram of a PC power plant is illustrated in Fig. 2.
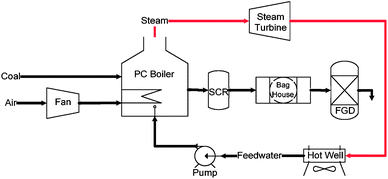 | ||
| Fig. 2 Simplified schematic diagram of a Pulverized Coal (PC) combustion process for power generation. | ||
In a PC power plant, coal is first pulverized into fine powder with over 70% of the particles smaller than 74 μm (200 mesh). The pulverized coal powder is then combusted in the boiler with the presence of ∼20% excess air.14 The heat of combustion is used to generate high pressure, high temperature steam that drives the steam turbine system based on a regenerative Rankine cycle for electricity generation. Although the underlying concept is quite simple, the following challenges need to be addressed for modern PC power plants: enhancement of energy conversion efficiency; effective control of hazardous pollutants emission; and CO2 capture (and sequestration).
2.1 Energy efficiency improvement
An increase in the combustion process efficiency leads to reduced coal consumption and hence, a potential cost reduction for electricity generation. The first generation coal fired power plants constructed in the early 1900s converted only 8% of the chemical energy in coal into electricity (based on the higher heating value, HHV).16 Since then, a significant improvement in plant efficiencies has been made. Thermodynamic principles require higher steam pressures and temperatures for a higher plant efficiency. The corrosion resistance of the materials for boiler tubes, however, constrains the maximum pressure and temperature of the steam. Most of the PC power plants currently under operation utilize sub-critical PC (Sub-CPC) boilers which produce steam with pressures up to 22 MPa and temperatures around 550 °C. The energy conversion efficiencies of traditional Sub-CPC power plants typically range from 33% to 37% (HHV).17 With an increase in the steam pressure, supercritical PC (SCPC) power plants were first introduced in the early 1960s in the US.16 Supercritical power plants involve steam with a typical pressure of ∼24.3 MPa and temperatures up to 565 °C, leading to a plant efficiency of 37 to 40%.17 Many supercritical power plants were constructed in the 1960s and 70s in the US. However, due to the low reliability of the boiler materials, the further application of the SCPC technology was essentially halted in the US in the early 1980s. The development of high performance super alloys coupled with increasing environmental concerns and the rising cost of coal during the last two decades has stimulated the revival of supercritical technology, especially in Europe and Japan, leading to the reduction of subcritical boilers in newly installed fleets.Recent advancements in coal combustion technologies are highlighted by the generation of “ultra-supercritical” (USCPC) steam conditions that can provide even higher process efficiencies. The ultra-supercritical condition refers to the “operating steam cycle conditions above 565 °C (>1050 °F)”.17 The pressure and temperature of the steam generated from existing ultra-supercritical power plants can reach 32 MPa and 610 °C, corresponding to an energy conversion efficiency of over 43%.17,18 The global on-going R&D activities on PC boilers focus on the development of super alloys that can sustain steam pressures up to 38.5 MPa and temperatures as high as 720 °C. It is expected that a plant efficiency of over 46% can be achieved under such conditions.17–19 Other efforts in ultra-supercritical technology include minimizing the usage of super alloys, improving the welding technique, and optimizing the boiler structure design to minimize the steam line to steam turbine.18
Besides PC boilers, Fluidized Bed Combustors (FBC) using either turbulent fluidized beds or circulating fluidized beds are also being used for steam and power generation world wide. In these processes, limestone is often injected to capture SOx formed during coal combustion. Compared to PC boilers, the FBC has lower SOx and NOx emissions.20,21 Furthermore, it has superior fuel flexibility.22 Most commercial FBC plants operate under atmospheric pressures, with energy conversion efficiencies similar to subcritical PC power plants. Higher efficiencies can be achieved by operating the FBC at elevated pressures.22–24 The Pressurized Fluidized Bed Combustor (PFBC) generates a high temperature, high pressure exhaust gas stream which drives a gas turbine–steam turbine combined cycle system for power generation. In an advanced PFBC (APFBC) configuration, fuel gas is generated from coal via particle oxidation and pyrolysis. The fuel gas is combusted to drive a gas turbine (topping cycle). Such a process has the potential to achieve an energy conversion efficiency of over 46%.23 To date, the PFBC demonstrations have shown relatively low plant availability. In addition, the capital investment for PFBC is higher than PC power plants with a similar efficiency.25 Other potential challenges to the PFBC technology include scale-up, high temperature particulates/alkali/sulfur removal for gas turbine operation, and mercury removal from the flue gas.22,26Table 1 compares the performance of different coal combustion technologies. The energy penalties for the 90% CO2 capture using a retrofit monoethanolamine (MEA) scrubber as discussed in Section 2.3 are also shown in Table 1.
| Technology | Sub-CPC | SCPC | USCPC | AFBC | PFBC/APFBC |
|---|---|---|---|---|---|
| a Percentage decrease in energy conversion efficiency when a retrofit MEA system is used to capture 90% of the CO2 in the flue gas.b Estimated based on ASPEN simulation by authors. | |||||
| Base case efficiency (%) HHV | 33∼37 | 37∼40 | 40∼45 | 34∼38 | 38∼45 |
| MEA retrofit derating (%)a | 30–42 | 24–34 | 21–30 | ∼35b | ∼30b |
2.2 Flue gas pollutant control methods
Modern coal combustion power plants need to be able to capture environmentally hazardous pollutants released from coal combustion. Such pollutants include sulfur oxides, nitrogen oxides, fine particulates, and trace heavy metals such as mercury, selenium, and arsenic. Methods for capturing these contaminants from the flue gas streams abound. The challenges, however, lie in the efficient and cost effective removal of these contaminants.The traditional method for SOx removal utilizes wet scrubbers with alkaline slurries. The wet scrubber is effective; however, it is costly and yields wet scrubbing wastes that must be disposed of. Alternative methods have included more cost effective lime spray drying and dry-sorbent duct-injection. The lime spray drying method employs slurry alkaline spray yielding scrubbing wastes in solid form, easing the waste handling. The dry-sorbent duct-injection employs a dry alkaline sorbent for direct in-duct injection, circumventing the use of the scrubber. The recent pilot testing using re-engineered limestone sorbents of high reactivity yields a sorbent sulfation efficiency of over 90%, compared to under 70% with ordinary limestone sorbent, indicating a viability of the dry-sorbent duct-injection method with very active sorbents.33–35 The NOx is commonly removed by selective catalytic reduction (SCR). Other methods that can be employed include low NOx burner and O3 oxidation. The recent pilot testing of the CARBONOx process using coal char impregnated with alkaline metal revealed a high NOx removal efficiency at low flue gas temperatures.36 The trace heavy metals such as mercury, selenium, and arsenic can be removed by calcium based sorbent and/or activated carbon.33,37
The techniques to control the flue gas pollutants indicated above are well-developed. An effective capture (and sequestration) of CO2, an important green house gas (GHG) that accounts for 64% of the enhanced green house effect38 is, however, a challenging task.
2.3 CO2 capture systems
Coal-fired power plants are responsible for nearly a third of all anthropogenic CO2 emissions.39 Therefore, cost-effective carbon capture technologies for these plants play an important role in CO2 mitigation.CO2 represents ∼15% of the atmospheric pressure flue gas stream from coal combustion power plant (dry basis). Low CO2 partial pressures combined with the extremely high flue gas generation rate make the CO2 capture from PC power plants an energy consuming step. An ideal CO2 capture technology would incorporate effective process integration schemes while minimizing the parasitic energy requirement for CO2 separation.
The existing CO2 capture techniques from PC power plants include the well-established MEA scrubbing technology. Fig. 3a shows the schematic diagram of the MEA scrubbing process which indicates the key stream conditions of the process.40–42 In this process, the flue gas is first cooled down to ∼40 °C before entering the absorber where fresh amine is used to absorb CO2 in the flue gas stream. The spent amine solution with a high CO2 concentration is then regenerated in the stripper under a higher temperature (100–150 °C), and CO2 is then recovered at low pressure (0.1–0.2 MPa). A large amount of high temperature steam is required to strip the CO2 in the regeneration step.42,43 Therefore, a significant amount of energy will be consumed for steam generation and the subsequent CO2 compression step. It is estimated that the CO2 capture (separation and compression) using amine scrubbing will reduce the power generated from the entire plant by as much as 42%,29 which amounts to ∼70–80% of the total cost in the overall three-fold carbon management steps, i.e. carbon capture, transportation, and sequestration.44,45 As a result, a process that can reduce the energy consumption in the CO2 capture step will be vital for CO2 management in coal fired power plants.
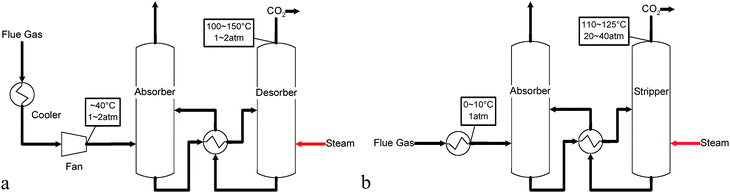 | ||
| Fig. 3 Conceptual schematic of (a) the MEA scrubbing technology for CO2 separation; (b) the Chilled Ammonia technology for CO2 separation. | ||
The chilled ammonia process, illustrated in Fig. 3b, is another solvent based CO2 capture technology where ammonia carbonate and bicarbonate slurries are used to capture the CO2 in the flue gas stream at 0–10 °C and atmospheric pressure. The CO2 rich solvent is then regenerated at 110–125 °C and 2–4 MPa. The capability to regenerate CO2 at elevated pressures reduces the energy consumption for CO2 compression. Based on the studies by the Electric Power Research Institute (EPRI) and ALSTOM, the overall energy penalty for CO2 capture is estimated to be lower than 16% when the chilled ammonia process is used.46,47 A 5 MWth (megawatts thermal) equivalent chilled ammonia process demonstration plant, jointly supported by ALSTOM and EPRI, is currently under construction at We Energies' Pleasant Prairie Power Plant in Wisconsin.48 American Electric Power (AEP) is also planning to demonstrate the chilled ammonia process at the 20 MWe (megawatts electricity) scale, starting in 2009, before building a 200 MWe commercial level chilled ammonia retrofit system in 2012.49
Similar to solvent based CO2 scrubbing techniques, high temperature sorbents such as limestone, potassium carbonates, lithium silicates, and sodium carbonates can be used to capture CO2 in the flue gas at elevated temperatures.50,51 With better heat integration, these strategies can potentially decrease the energy consumption in the CO2 separation step. One scheme for heat integration is based on the calcium based carbonation–calcination reaction (CCR) process which uses hydrated lime, and natural or re-engineered limestone sorbents at 600–700 °C for CO2 separation.52Fig. 4 delineates the heat integration strategies for retrofitting the CCR process to an existing PC power plant.
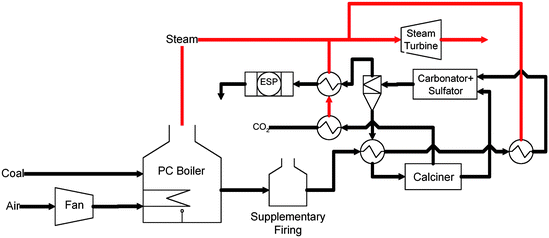 | ||
| Fig. 4 Conceptual schematic of Carbonation–Calcination Reaction (CCR) process integration in a 300 MWe coal fired power plant depicting heat integration strategies. | ||
In the CCR process, both CO2 and SO2 in the flue gas are captured by the CaO sorbent in the carbonator operated at ∼650 °C, forming CaCO3 and CaSO3/CaSO4 The carbonated sorbent, CaCO3, is then regenerated to calcium oxide (CaO) sorbent in the calciner at 850–900 °C, yielding a pure CO2 stream. The sulfated sorbent and fly ashes are removed from the system by means of a purge stream. Due to an optimized energy management scheme, the CCR process consumes 15–22% of the energy generated in the plant.53,54 The process is being demonstrated in a 120 kWth (kilowatts thermal) pilot plant located at The Ohio State University (OSU). A similar process is being demonstrated at CANMET Energy Technology Center in Canada.55 Studies or reviews on the post combustion CO2 capture using solid sorbents can be found in other literature sources.56–59
In addition to the absorption–adsorption based technologies, oxy-fuel combustion technology provides another means for carbon management in coal fired power plants. In this technology, pure oxygen instead of air is used for coal combustion. As a result, a concentrated CO2 stream is generated, avoiding the need for CO2 separation. However, the energy-consuming cryogenic air separation step will reduce the overall plant efficiency by 20–35%.17,30,45,60 This process has been successfully demonstrated by the Babcock & Wilcox Company on a 1.5 MWth pilot scale PC unit. Demonstration on a 30 MWth unit is currently under way. The on-going pilot scale studies on oxy-fuel combustion include those carried out by ALSTOM, Foster-Wheeler, CANMET Energy Technology Center, Vattenfall, and Ishikawajima-Harima Heavy Industries (IHI).61
To generalize, a number of retrofit systems under different stages of development can be used to capture CO2 from existing power plants. Since PC power plants will continue to provide a significant portion of the electricity needs well into the 21st century,7 these CO2 capture systems are essential to mitigate the environmental impact from coal burning. In general, however, CO2 capture and compression from a coal combustion flue gas is costly and energy intensive. A more promising approach to reduce the overall carbon footprint of a coal based plant is to adopt coal conversion processes that are intrinsically advantageous from a carbon management and energy conversion standpoint. Among the various options, coal gasification described below offers such attraction.
3. Coal gasification processes
For years, the commercial efforts on clean coal processes have been centered on coal combustion for power generation. However, new process developments with a focus on higher energy conversion efficiencies for electricity generation as well as variability in product formation have generated considerable interest. Coal gasification schemes can provide a variety of products—e.g. hydrogen, liquid fuels and chemicals—besides electricity. Further, gasification is a preferred scheme from a pollutant and carbon management viewpoint.3.1 Overview
Compared to combustion, coal gasification is relatively new. Commercial gasification processes date back to the late 18th century when coal was converted into town gas for lighting and cooking. Since the 1920s, the gasification process has been used to produce chemicals and fuels.62 Unlike traditional combustion processes which fully oxidize carbonaceous fuels to generate heat, modern coal gasifiers convert coal into syngas via partial oxidation reactions with oxygen or with steam and oxygen under elevated pressures.14,62 The high pressure syngas stream, undiluted by N2 in the air, has a much lower volumetric flow rate when compared to that of the flue gas from coal-fired power plants. As a result, the partial pressure of the contaminants is significantly increased. For instance, the volumetric flow rate of syngas generated from a dry feed, oxygen blown gasifier can be two orders of magnitude lower than that from a PC boiler with similar coal processing capacity (dry basis). Meanwhile, the partial pressure of CO2 in the syngas after the water gas shift (WGS) reaction can be 80 times higher than that in the PC boiler flue gas (dry basis). The significantly reduced gas flow rate and increased gas partial pressures make the pollutant and CO2 control an easier task for gasification processes when compared to coal combustion processes.Fig. 5 shows the modern coal gasification process that generates a variety of products. In the coal gasification process, coal first reacts with oxygen (and steam) to produce raw syngas. The raw syngas, with pollutants such as particulates, H2S, COS, HCl, ammonia, and mercury, is purified before it is sent to a gas turbine–steam turbine combined cycle system for electricity generation. This syngas route is known as the Integrated Gasification Combined Cycle (IGCC). The electricity generation efficiency of the IGCC process can be higher than 45% (HHV) without CO2 capture.62,64 In a carbon constrained scenario, however, the CO in the syngas stream will be further converted to CO2 and H2 through the water–gas shift (WGS) reaction:
| CO + H2O → CO2 + H2 | (1) |
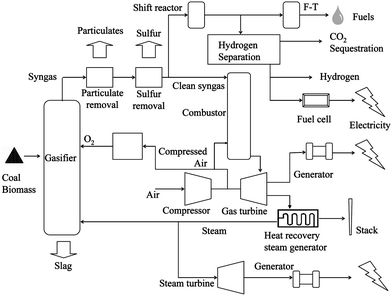 | ||
| Fig. 5 Schematic diagram of coal gasification processes.63 | ||
Thus, the resulting gas stream contains a high CO2 concentration (up to ∼40% by volume on the dry basis). The CO2 (and H2S) can be captured using either chemical absorption based acid gas removal processes such as monoethanolamine (MEA) or methyldiethanolamine (MDEA) described in Section 2.3 or physical absorption based processes such as Selexol and Rectisol, yielding concentrated H2.65 The H2 can be used to generate electricity through a combined cycle system with minimal carbon emissions.
Alternatively, the H2 stream can be further purified using pressure swing adsorption (PSA) units. The resulting high-purity H2 can be used for fuel cell applications. Besides electricity and H2 generation, syngas can also be converted to chemicals and liquid fuels such as diesel and naphtha through the Fischer–Tropsch (F–T) reactions, which can be represented by:66–68
| (2n + 1)H2 + nCO → CnH2n + 2 + nH2O | (2) |
The process that converts coal to liquid fuels via coal gasification and F–T synthesis is also referred to as the indirect coal-to-liquid (CTL) process. Unlike the indirect CTL process, the direct CTL process liquefies coal directly by reacting it with hydrogen at elevated pressure.69,70 The direct CTL process can achieve a high liquid fuel yield—close to 3 barrel liquids per ton of coal when coal is also used as the hydrogen source.71 The pilot demonstrations of CTL processes took place during the 1970s to 1990s and included the H–Coal process (by Hydrocarbon Research Inc.) and the Integrated Two-Stage Liquefaction process (by Lummus Company).71 The first commercial direct CTL process plant is being built in Inner Mongolia, China by Shenhua Group Corporation. The production cost for the direct CTL process is higher than for the indirect CTL process.71
Processing of coal using the gasification approach has the advantages in product versatility and pollutant controllability when compared to the combustion approach. However, gasification is more capital intensive. A study conducted in 2001 indicated that an IGCC system required 6–10% more capital investment when compared to an ultra-supercritical PC plant.72 Both plants have similar energy conversion efficiencies. Although CO2 capture from the gasification process is easier when compared to a PC plant, the CO2 capture, nevertheless, represents an energy and capital intensive step in the process. The CO2 capture can derate the energy conversion efficiency of the IGCC system by 13–24%, increasing the cost of electricity by 25–45%.28,31,73–75 Other issues related to gasification include large parasitic energy consumption in the WGS step due to the need for the excessive steam as well as the temperature and pressure swing requirement in the process for sulfur and mercury removal.
Gasification, like other technologies, has undergone evolution since its inception. Over the years, different types of gasifiers have been developed which provide a higher carbon conversion, cold gas and thermal efficiencies, and flexibility in the type of fuel used. These gasifier types include the fixed/moving bed gasifier, fluidized-bed gasifier, entrained-flow gasifier, and transport gasifier.62 Most of the modern gasifiers adopt an entrained-flow design due to better fuel flexibility, carbon conversions, and syngas quality.76 Other ongoing research activities include the use of an Ion Transport Membrane (ITM) instead of the cryogenic separation technique to reduce the energy consumption of the air separation unit (ASU),77,78 the increase in the gas turbine inlet temperature to increase the combined cycle efficiency, and the development of a warm and hot gas clean up system to efficiently remove pollutants such as particulates, sulfur and mercury.79–81
As there is a large degree of operational variation in individual units and in an integrated process system, optimization of the gasification process requires elaborate consideration of all the viable process configurations. For this purpose, simulation software such as ASPEN Plus® is often used to aid in the analysis of the process configurations under various process variables. In the following section, a case study is presented which illustrates the energy conversion efficiency for an IGCC system with CO2 capture through simulation using the ASPEN® plus software.
3.2 ASPEN analysis on IGCC system with CO2 capture – a case study
Aspen Plus® has been widely used to simulate energy conversion systems.41,82–87 Based on appropriate assumptions and relevant experimental data of the individual units, the ASPEN Plus® software can assist in the evaluation of the process performance, and in the optimization of the process configuration. The IGCC system illustrated in this case study uses a GE/Texaco slurry-feed, entrained flow gasifier with total water quench syngas cooler. The flow diagram of the process is shown in Fig. 6.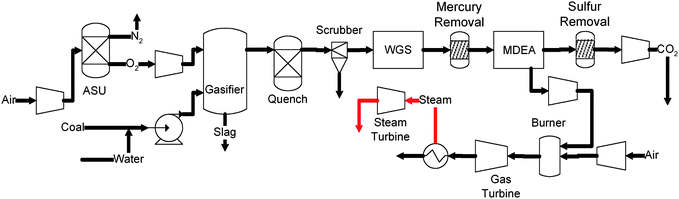 | ||
| Fig. 6 IGCC process with CO2 capture. | ||
In this process, coal is first pulverized and mixed with water to form coal slurry. The coal slurry is then pressurized and introduced to the gasifier to be partially oxidized at 1500 °C and 3.04 MPa (30 atm). The high temperature raw syngas after gasification is then quenched to 250 °C with water. The quenching step solidifies the ash. Moreover, most of the NH3 and HCl in the syngas are removed during this step. After quenching, the syngas is sent to a Venturi scrubber for further particulate removal. The particulate-free syngas, saturated with steam, is then introduced to the sour WGS unit. The syngas exiting the WGS unit contains mainly H2 and CO2 with small amount of CO, H2S, and mercury. This gas stream is then cooled down to 40 °C and passed through an activated carbon bed for mercury removal. The CO2 and H2S in the syngas are then removed using an MDEA unit, resulting in a concentrated hydrogen stream with small amounts of CO2 and CO. The hydrogen rich gas stream is then compressed, preheated, and combusted in a combined cycle system for power generation. The combined cycle system consists of a gas turbine with an inlet firing temperature of 1430 °C and a two stage steam turbine working at 550 °C and 3.55 MPa (35 atm). The CO2 obtained from the MDEA unit is compressed to 15.20 MPa (150 atm) for sequestration.
ASPEN modeling on coal conversion systems has been extensively discussed in various literature.41,82,84–87 The following section briefly recapitulates the key steps to set up an ASPEN simulation model on the IGCC system described above.
Prior to the simulation, a representative process flow sheet that contains all the major units is developed (Fig. 6). The appropriate assumptions for the simulation are then determined. The key assumptions are listed as follows:
■ 132.9 tonne/h of Illinois #6 coal is fed into the system (1000 MW in HHV)
■ Energy consumed for units such as acid gas removal are simulated based on performance data of the commercial units
■ The GE slurry feed gasifier has a carbon conversion of 99%, heat loss in the gasifier is 0.6% of the HHV of coal
■ A GE 7H gas turbine combined cycle system is used, all the exhaust gas is cooled down to 130 °C before exiting the Heat Recovery Steam Generator (HRSG)
■ At least 90% of the CO2 generated needs to be captured and compressed to 15.20 MPa (150 atm) for sequestration
■ The mechanical efficiency of pressure changers is 1, whereas the isentropic efficiency is 0.8∼0.9
In order to accurately simulate the individual unit in the flow sheet, appropriate ASPEN Plus model(s) for each unit is determined. These models are listed in Table 2.
| Unit operation | Aspen Plus model | Comments / specifications |
|---|---|---|
| Air separation unit | Sep | Energy consumption of the ASU is based on specifications of commercial ASU/compressors load. |
| Coal decomposition | Ryield | Virtually decompose coal into various components (pre-requisite step for gasification modeling) |
| Coal gasification | Rgibbs | Thermodynamic modeling of gasification |
| Quench | Flash2 | Phase equilibrium calculation for cooling |
| WGS | Rstoic or Rgibbs | To simulate conversion of WGS reaction based on either WGS design specifications or thermodynamics |
| MDEA | Sep or Radfrac | Simulation of acid gas removal based on design specifications |
| Burner | Rgibbs or Rstoic | Modeling of H2/syngas combustion step |
| HRSG | MHeatX | Modeling of heat exchanging among multiple streams |
| Gas compressors | Compr or Mcompr | Determines power consumption for gas compression |
| Heater and cooler | Heater | Simulates heat exchange for syngas cooling and preheating |
| Turbine | Compr | Calculates power produced from gas turbine and steam turbine |
Aspen Plus® has a comprehensive physical property database. Therefore, most of the chemical species involved in the process can be selected directly from the build-in database. The nonconventional components such as coal and ash can be specified conveniently using the general coal enthalpy modulus embedded in the ASPEN software. After the chemical species in the process are defined, the related physical property methods are selected according to the simulator's category. In this simulation, the global property method is PR–BM, whereas local property methods are specified whenever necessary.
The ASPEN model is finalized by establishing detailed operating parameters based on the operating conditions and design specifications of the individual unit. The units are then connected in the same arrangement as shown in the flow sheet. An appropriate convergence setting is determined to ensure accurate simulation results. Table 3 generalizes the simulation results of the IGCC system described above.
| Thermal energy input (MWth) | Parasitic energy consumption (MWe) | Power generation (MWe) | Net power (MWe) | ||||
|---|---|---|---|---|---|---|---|
| Coal | ASU | CO2 removal | CO2 compression | Gas turbine | Steam turbine | ||
| IP | LP | ||||||
| 1000 | 39.4 | 9.4 | 17.7 | −249.1 | −86.4 | −79.1 | −348.1 |
The results shown in Table 3 can replicate the performance of existing IGCC power plants reported by Higman.62 The ASPEN simulation can be effective for evaluating the performance of various coal conversion systems based on a common set of assumptions.
4. Advanced coal conversion processes
Although with various improvements, as discussed in section 3, the efficiency of the conventional gasification systems is still limited due to the elaborate steps needed such as syngas cleaning and conversion, and gas separation and compression. Advanced coal conversion processes, which adopt novel process intensification strategies, streamline the conversion processes thereby yielding high energy conversion efficiency. Such techniques, which are currently at various stages of demonstration, encompass the membrane based approach and the chemical looping based approach. Both approaches can process syngas derived from coal or any other carbonaceous feedstock. The chemical looping approach can also process coal or other carbonaceous feedstock directly. These approaches are elaborated below.4.1 Membrane based gasification systems
A membrane is a selective barrier between two phases. The molecules or small particles can transport from one phase to the other through the membrane. A H2 or CO2 selective membrane can be utilized in gasification processes to reduce the energy penalty for CO2 capture and to enhance the hydrogen/electricity generation.The selective nature of a membrane can be attributed to one or more of the following mechanisms: (a) Knudson diffusion; (b) surface diffusion; (c) capillary condensation; (d) molecular sieving; (e) solution diffusion; and (f) facilitate transport.88 As the smallest diatomic molecule, hydrogen can be separated from other gaseous species involved in the coal gasification process based on all the mechanisms stated above. On the other hand, most CO2 selective membranes are based on either solution diffusion or facilitate transport mechanism since the CO2 molecule is significantly larger. An amine based carrier is often used to facilitate the transportation of CO2 from the retentate side to the permeate side.88,89 As a result, a hydrogen selective membrane can be made of metallic, inorganic (ceramic), porous carbon, polymer, or hybrid materials while most of the CO2 selective membranes for separating CO2 from hydrogen are polymeric.
The desirable features of a membrane include good permeability, selectivity, reliability, and tolerance to contaminants. For commercial applications in gasification processes, it should also be affordable, thermally stable, and durable. Of all the H2-selective membranes, metallic membranes and ceramic membranes are the most extensively studied.90–95
The metallic H2-selective membranes generally have a very high selectivity and thermal stabilities. The potential candidates include palladium, platinum, tantalum, niobium, and vanadium.88,90,93 Among these metals, Pd-based membranes, although relatively costly, have demonstrated the highest selectivity and good permeability and thermal stability. However, the presence of hydrogen at below 300 °C can cause the embrittlement of the Pd-based membrane due to the Pd–H phase transition. In order to reduce the membrane degradation as well as to reduce the cost, Pd-based membranes are often alloyed with Ag, Au, Y, Cu, or Se. These alloys are processed into a layer as thin as blow 1 μm and then doped on top of a porous ceramic or metallic support.94,96 By alloying and supporting, the usage of Pd is minimized with increased physical strength of the membrane.94 One major challenge to Pd-based membranes is that the presence of sulfur compounds such as H2S and COS under elevated temperatures can poison the Pd-based membranes. Recent studies indicate that alloying can increase the sulfur tolerance of the membrane.97 However, a high sulfur content that is close to or beyond the thermodynamic limit for the formation of stable sulfides will nevertheless deactivate the membrane.92 In addition, when ceramic support is used in the Pd-based metallic membrane, it will be necessary to resolve such issues as the mechanical strength of the support and the large difference in thermal expansion coefficients between the metallic membrane and the ceramic support. For metallic support, the challenge lies in the stability of the crystal structure due to inter-metallic diffusion. Therefore, desirable improvements in the Pd-based membrane for gasification applications include further reduction in cost coupled with increased durability, sulfur tolerance, and H2 flux. Besides the Pd-based metallic membranes, non Pd-based alloys98 and amorphous metals93,99 are also under investigation with the prospect of developing less costly metallic membranes with satisfactory performance.
Ceramic H2-selective membranes such as porous silica- and zeolite-based membranes represent another category of promising hydrogen separation materials.100–102 Both membranes are microporous inorganic membranes comprised of a membrane layer, an intermediate layer, and a support. These membranes have several advantages when compared to metallic membranes including low cost, ease of fabrication, and less susceptibility to H2 embrittlement. Moreover, very high hydrogen permeability can be achieved using an ultra-thin amorphous silica membrane. However, improvements that need to be made in these membranes include selectivity, defect reduction, thermochemical stability and operational stability. Table 4 generalizes the performances of existing H2-selective membranes as compared to the 2010 performance target set by the US DOE.
Although zeolite-based membranes can be used to selectively remove CO2 from other gases such as N2 and CH4 based on adsorption preference,105,106 very limited studies have been performed on the separation of CO2 from H2 using such membranes.51 Other attempts include those performed by Air Products and Chemical Inc. that use nanoporous carbon-based membranes to separate CO2 from the tail gas of the Pressure Swing Adsorption (PSA) unit.107,108 However, these membranes have relatively low CO2 selectivity over H2.107–109 To date, most CO2-selective membranes for separating CO2 from H2 are polymeric membranes based on either solution diffusion mechanism or facilitate transport mechanism.89,110–113 The challenges to the polymeric CO2-selective membranes include limited operating temperature and relatively low CO2/H2 selectivity and flux.
As mentioned in Section 3, the WGS reactor(s) and the CO2 separation units consume a significant amount of parasitic energy for the coal to hydrogen process and IGCC process with CO2 capture. The applications of the H2- or CO2-selective membranes in coal gasification systems for the intensification of the CO shift and hydrogen purification steps have been extensively studied during the last decade.
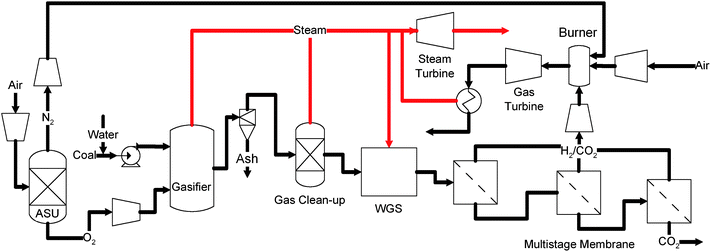
Several different configurations using different types of membranes have been investigated, exhibiting promising results. Fig. 7 shows a multi-stage membrane system that recovers CO2 from a shifted syngas stream proposed by Kaldis et al.114 In this process, the clean syngas stream resulting from the coal gasifier and gas cleanup units is first shifted in a series of WGS units, resulting in a gaseous mixture consisting mainly of H2, CO2, CO, and N2. The mixed gas is then introduced to a series of H2-selective membranes to recover a concentrated CO2 stream on the retentate side. The permeate side, with concentrated hydrogen, is combusted in the gas turbine for electricity generation. The performances of both polymer and ceramic membranes are investigated using ASPEN Plus® simulations. The results indicated that the CO2 emission can be reduced by over 50% using the multi-stage membrane system but with 17–28% parasitic energy consumptions.114
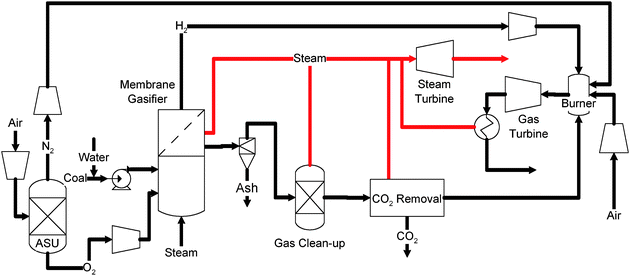
More advanced membrane systems integrate the function of both WGS and CO2 separation using either H2- or CO2-selective membranes. Such configurations are shown in Figs. 8a and b.63,92,115–120
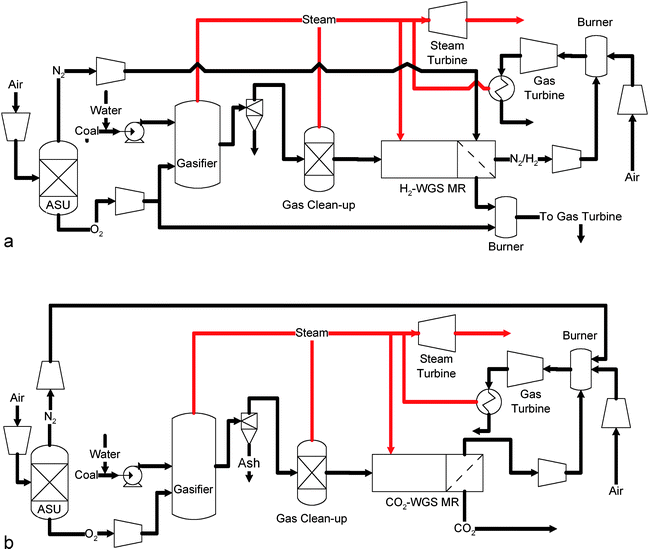 | ||
| Fig. 8 (a) Schematic of H2-selective membrane enhanced IGCC process.118 (b) Schematic of CO2-selective membrane enhanced IGCC process.118 | ||
Fig. 8a illustrates a specific configuration when a H2-selective membrane is used.63,92,115,117–119 In such a configuration, a conventional gasifier and a gas clean up system is used to produce clean syngas. The clean syngas is then sent to the membrane–WGS reactor. The membrane–WGS reactor has two compartments, i.e. reaction side and product side. The two compartments are segregated by a semi-permeable membrane that is selective to hydrogen. In the reaction side, the CO in the syngas is converted to H2 and CO2via WGS reaction. The H2 produced in the reaction side is continuously permeated through the membrane to the product side. As a result, a high purity H2 product can be obtained without engaging traditional separation techniques. Such hydrogen can either be used as a product or combusted with air for power generation. In addition, due to the removal of the hydrogen product, the WGS reaction, which is limited by thermodynamic equilibrium, can be enhanced. The tail gas from the reaction side, with a high CO2 concentration mixed with residual CO and H2, is combusted in a combined cycle system with O2 to generate electricity. The resulting CO2 is then sequestered.
The underlying principle for the membrane-based system shown in Fig. 8b116,118 is similar to that of the system shown in Fig. 8a. The only difference lies in the type of membrane used for separating the shifted syngas. In this configuration, a CO2-selective membrane is used to divide the reaction side and the product side in the membrane reactor. As a result, the CO2 rather than the H2 will be transferred from the reaction side to the product side. The simultaneous removal of CO2, which is another product of the WGS reaction, can also enhance the reaction. The CO2 stream in the product side, swept by steam, can be directly sequestered while the H2-rich stream in the reaction side can either be purified to obtain a hydrogen product or combusted with air for power generation.
Extensive studies have been performed to analyze the performance of gasification processes integrated with membrane systems. Chiesa et al. (2007)119 indicated that although a significant energy penalty has to be paid for CO2 capture, a Pd-based membrane system such as that shown in Fig. 8a is thermodynamically advantageous when compared to commercial WGS–CO2 capture systems. A process analysis carried out by Amelio et al. (2007)115 indicated that if integrated with an IGCC system using a GE gasifier, an energy penalty around 17.5% (46.0% before capture to 39.3% HHV after capture) will incur when a Pd-based H2-selective membrane system is used to capture 90% of the CO2. Grainger et al. (2008)116 studied the performance of a CO2-selective polyvinylamine membrane in an IGCC system identical to the Puertollano plant. The results revealed a 22.9% energy penalty for 85% CO2 capture. Carbo et al.118 compared the performance of a H2-selective membrane system to that of a CO2-selective membrane in an IGCC process with an entrained flow, oxygen blown gasifier. The results indicated that the energy penalty is merely 11.2% when a H2-selective membrane is used for 100% CO2 capture. In contrast, a 19.4% energy penalty will incur when a CO2-selective membrane is used for 90% CO2 capture. The selectivity of both membranes was assumed to be infinity in this study.
A more advanced approach integrates a H2-selective membrane into the gasifier for H2 generation (Fig. 9).121 In this case, a membrane is installed in the coal gasifier to separate out the hydrogen generated. The rest of the syngas is combusted with oxygen for power generation. Such a process, although potentially more efficient, requires a membrane that tolerates ultra-high temperatures and various contaminants. The development of such high performance membranes may not be feasible in the near future.
To generalize, although the membrane systems can not eliminate the energy penalty for CO2 capture in gasification plants, they have the potential to reduce such a penalty when compared to the traditional approach. The parasitic energy consumed for CO2 capture using a membrane-based system lies in the need for gas compression, and in some cases, the generation of extra oxygen to combust the CO2 rich tail gas and the need for extra steam as sweep gas. It is also worth noting that from the economic standpoint, the membrane-WGS reactor can replace both the shift unit and CO2 separation unit. Therefore, notable cost reduction can be realized provided that a membrane with good reliability and durability can be mass-produced at a reasonable cost.
4.2 Chemical looping based gasification systems
As discussed in Section 4.1, membrane-based systems intensify the syngas conversion scheme by integrating the CO shift and the CO2 removal step. Due to the limited tolerance of membranes towards pollutants such as sulfur and halogen compounds, the raw syngas from the gasifier needs to be extensively cleaned before entering the membrane system. Chemical looping based systems have the potential to simplify the syngas cleaning procedures. Moreover, the pressure drop due to the membrane separation can be reduced in chemical looping systems.The chemical looping strategy that generates the end products with the aid of chemical intermediates through a series of reaction schemes was proposed many years ago. One example is the steam-iron process used for commercial hydrogen production from coal derived producer gas in the early 20th century.122,123 Another example is the CO2 generation, reported a half-century ago, for the beverage industry using the chemical looping process with the oxides of copper or iron as the looping particles.124,125 Although the adoption of the chemical looping strategy in the early years was mainly prompted by the lack of effective chemical conversion/separation techniques in the product generation, modern applications of chemical looping processes are prompted by the need for developing an optimized reaction scheme that minimizes the exergy loss involved in the chemical/energy conversion system.126–128 Also driven by the envisaged CO2 emission control, the recent development in chemical looping systems have focused on the efficient conversion of gaseous carbonaceous fuels such as natural gas and coal derived syngas,53,128–131 and solid fuels such as petroleum coke and coal132,133 while separating CO2 readily through the looping reaction scheme. In this section, chemical looping systems using coal derived syngas will be discussed. Looping systems that directly convert coal will be presented in Section 4.3.
In this section, two types of chemical looping based approaches that enhance the performance of the coal gasification processes are given. Type A chemical looping such as the Syngas Chemical Looping Combustion (Syngas–CLC) processes and the Syngas Chemical Looping (SCL) process use oxygen carrier particles, typically metal oxides, to convert coal derived syngas, whereas Type B chemical looping such as the Calcium Looping Process (CLP) and the Thermal Swing Sorption Enhanced Reaction (TSSER) process utilize solid CO2 sorbents to enhance the syngas conversions.
Syngas chemical looping combustion. Fig. 10 shows a typical chemical looping combustion process using coal derived syngas as feedstock. As can be seen, coal is first gasified into raw syngas. A set of gas cleanup units is then used to remove the contaminants to a level below the tolerance limit of the oxygen carrier particle used in the process. The cleaned syngas then reacts with the oxygen carrier particles in the first reactor which is noted as the reducer or the fuel reactor. The main reactions in this reactor are:
| MeO + H2 → Me + H2O | (3) |
| MeO + CO → Me + CO2 | (4) |
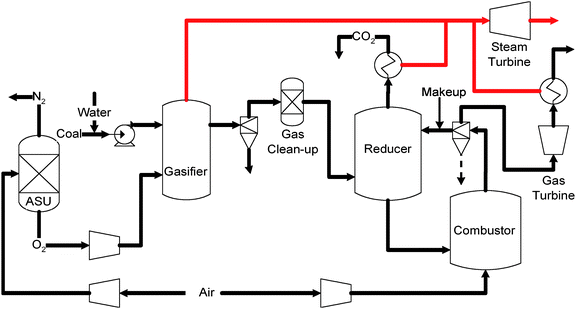 | ||
| Fig. 10 Schematic flow diagram of syngas chemical looping combustion processes. | ||
As can be seen from reaction 3 and 4, the syngas is oxidized to CO2 and steam by the metal oxide particles before exiting the reducer. A concentrated CO2 stream can then be readily obtained by condensing out the steam in the reducer. The CO2 stream can be further pressurized and transported for sequestration. Meanwhile, the reduced metal oxide particles will be introduced to the second reactor, i.e. the combustor or the air reactor, to react with air:
| Me + 1/2O2(Air) → MeO | (5) |
The oxidization reaction in the combustor is highly exothermic. As a result, a high temperature, high pressure, oxygen depleted exhaust gas stream is generated from the combustor. Such an exhaust gas stream is used to drive a combined cycle system for electricity generation. Meanwhile, the particles, fully regenerated by air, are recycled to the reducer for another redox (reduction–oxidation) cycle.
In the CLC process, the coal derived syngas is combusted with air indirectly through the looping particles, i.e., metal oxide. Hence, the fuel combustion products, i.e., CO2 and steam are not diluted by nitrogen in the air, and the CO2 separation from nitrogen is, therefore, avoided. Moreover, the syngas cleanup steps can potentially be simplified since the metal oxide particles can be more robust towards contaminants when compared to membranes.85 As a result, the acceptable level of the contamination in the syngas for chemical looping processes can be higher than the membrane based systems. An additional advantage for the looping system is that the difference between the pressure of the concentrated CO2 exhaust and that of the syngas feedstock is merely the pressure drop of the reducer, which can be significantly lower than the pressure drop in the solvent-based and membrane-based CO2 separation system.
The focal areas of the research and development activities on the CLC processes are on the oxygen carrier particle design and synthesis, looping reactor design and operation, and looping process analysis and demonstration. Various types of oxygen carrier particles, including the oxides of Ni, Fe, Mn, Cu, and Co, have been investigated for syngas chemical looping combustion.129,140–142 Most of the studies focus on developing particles that maintain good reactivity for multiple redox (reduction–oxidation) cycles. Other factors being considered include particle strength improvements and carbon deposition reduction. In order to obtain particles with the desirable properties, ceramic materials are often used to support the oxygen carrier. These supporting materials include alumina, MgAl2O4, yttria-stabilized zirconia (YSZ), TiO2, bentonite, and barium–hexaaluminate (BHA). Metal oxide particles that can sustain multiple redox cycles in atmospheric reactor systems have been successfully synthesized. Important areas that need to be further explored include the pollutant tolerance of the particles and particle reactivity under elevated pressures. For instance, experiments in a high pressure TGA indicated that an increase in total pressure may have negative effects on the reduction rates of Cu, Ni, and Fe based oxygen carriers.143 This finding, however, was inconsistent with that obtained by Siriwardane et al. (2007) using NiO supported on bentonite.141 Jin and Ishida (2004) studied the pressure effect on the reactivity of NiO supported on MgAl2O4 under 0.1–0.91 MPa (1–9 atm) using a fixed bed reactor.129 They found that an increased carbon deposition under elevated pressures, which was consistent with that predicted from thermodynamic principles.85 An increased oxidation reaction rate was also observed under higher pressure by Jin and Ishida (2004);129 however, the pressure effect on the reduction reaction rate was not reported.
The syngas CLC process was tested in a 300 Wth (watts thermal) circulating fluidized bed chemical looping combustor at Chalmers University in Sweden.144–146 Different types of oxygen carrier particles including NiO supported on MgAl2O4,144 Fe2O3 supported on Al2O3,147 and Mn3O4 supported on Mg stabilized ZrO2148,149 have been used, yielding 99% or higher syngas conversions. Other CLC testing facilities include the 10 kWth circulating fluidized bed unit at Chalmers University,130 the 50 kWth circulating fluidized bed unit at Korea Institute of Energy Research (KIER),150 and the 120 kWth circulating fluidized bed unit at Vienna University of Technology.151 The published experimental results obtained from these testing facilities focus on the conversion of methane.
Both thermodynamic and ASPEN® plus simulations have been performed for the chemical looping combustion systems with syngas as feedstock. The exergy analysis conducted by Anheden and Syedberg (1998) indicated that when a CLC system with a Fe2O3-based oxygen carrier particle is used to a retrofit IGCC plant, a 7.8% increase in exergetic efficiency compared to a base case can be realized (from 45.19 to 48.72%).126 In their study, however, the energy for CO2 compression was not considered. The ASPEN® simulation conducted by Xiang et al. (2008) indicated that the gasification–CLC system has the potential to achieve 43.2% (LHV) efficiency for electricity generation with 99% CO2 captured.134
The performance of the syngas chemical looping combustion processes is dependent on two closely related factors, i.e. the oxygen carrier particle performance and the reactor design. Many research efforts on the CLC system have focused on the development of reactive and recyclable particles, given that fluidized bed reactors are to be used as the looping reactors. In fact, various factors need to be considered in selecting a particle, i.e., particle oxygen carrying capacity, reactivity, recyclability, cost, physical strength, oxygen carrying capacity, contaminant tolerance, melting points, and environmental effects. On the looping reactor, the use of fluidized bed reactor is evidenced by extensive on-going studies of high density circulating fluidized bed systems in which the riser serves as the combustor and the downer in bubbling or turbulent mode of operation serves as the reducer in chemical looping combustion applications.44,144,149,152 It should be noted, however, that reactor design can have a significant effect on particle conversion, and hence the process efficiency. Table 5 illustrates the effect of the flow pattern, i.e., fluidized bed or countercurrent moving bed in the fuel reactor on the solid particle conversion when a Fe based oxygen carrier particle is used. The results given in the table are based on the thermodynamic analysis and the assumptions presented in the table.
| Reactor type | Countercurrent moving bed | Fluidized bed/CSTRa |
|---|---|---|
a To account for back mixing in the fluidized bed reducer, the fluidized bed reactor is considered as CSTR.b Maximum metal oxide reduction at 850 °C when more than 99.9% syngas (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 2 H2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is converted. Results were obtained based on thermodynamic analysis.153c Effective oxygen carrying capacity = Maximum oxygen carrying capacity × Metal oxide loading × Maximum theoretical metal oxide conversion (metal oxide loading is 70% in this case). 1) is converted. Results were obtained based on thermodynamic analysis.153c Effective oxygen carrying capacity = Maximum oxygen carrying capacity × Metal oxide loading × Maximum theoretical metal oxide conversion (metal oxide loading is 70% in this case). | ||
| Oxygen carrier | Fe2O3 | Fe2O3 |
| Maximum metal oxide conversionb (%) | 51.5 | 11.27 |
| Effective oxygen carrying capacityc (wt %) | 10.82 | 2.37 |
| CO + H2 concentration in gas exhaust (%) | 0.005 | 0.005 |
It is seen that the theoretical solid conversion in the moving bed is nearly five times higher than that in the fluidized bed, resulting in significantly reduced solid circulation rate for the moving bed design and hence minimized reactor volume. Thus, for a successful CLC system operation, flow pattern consideration for the reactor is deemed important.
Syngas chemical looping gasification. Compared to the CLC processes, the Syngas Chemical Looping (SCL) process has the flexibility to co-produce hydrogen and electricity.131,137–139Fig. 11 shows a simplified block diagram of the SCL process developed at the Ohio State University.
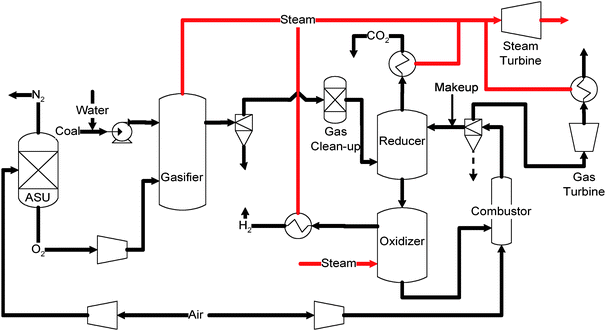 | ||
| Fig. 11 Simplified schematic of the Syngas Chemical Looping process. | ||
The SCL process can convert syngas with moderate levels of HCl, NH3, sulfur, and mercury; therefore, existing hot gas cleanup units (HGCU) will be adequate for raw syngas cleaning. The raw syngas exiting the HGCU will be introduced to the reducer, which is a moving bed of specially tailored iron oxide composite particles operated under a pressure similar to that of the syngas. In this reactor, the syngas is completely converted into carbon dioxide and water while the iron oxide composite particles are reduced to a mixture of Fe and FeO under 750–900 °C:
| Fe2O3 + CO → 2FeO + CO2 | (6) |
| FeO + CO → Fe + CO2 | (7) |
| Fe2O3 + H2 → 2FeO + H2O | (8) |
| FeO + H2 → Fe +H2O | (9) |
Similar to the CLC processes, an exhaust stream with concentrated CO2 can be obtained from the reducer. The contaminants in the syngas will also exit the reducer with the CO2 stream without attaching to the particle. These contaminants can be compressed and sequestered along with CO2 if allowed by regulation. As a result, the gas cleaning procedures are greatly simplified.
The Fe/FeO particles leaving the reducer are then introduced into the oxidizer which is operated at 500–750 °C at a pressure similar to that of the reducer. In the oxidizer, the reduced particles react with steam to produce a gas stream that contains solely H2 and unconverted steam. The steam can be easily condensed out to obtain a high purity H2 stream. The reactions involved in the oxidizer include:
| Fe + H2O (g) → FeO + H2 | (10) |
| 3FeO + H2O (g) → Fe3O4 + H2 | (11) |
The steam used in the oxidizer is produced from the heat released from syngas cooling and reducer/oxidizer exhaust gas cooling. In the SCL process, the oxidizer is slightly exothermic while the reducer can either be slightly exothermic or slightly endothermic depending on the syngas composition. Therefore, both reducer and oxidizer are operated under the adiabatic conditions. Heat is provided to or removed from the reactors by the oxygen carrier particles and the exhaust gas. The Fe3O4 formed in the reducer reactor is regenerated to Fe2O3 in an entrained flow combustor which also transports solid particles discharged from the oxidizer to the reducer. A portion of the heat produced from the oxidation of Fe3O4 to Fe2O3 can be transferred to the reducer through the particles:
| 4Fe3O4 + O2 → 6Fe2O3 | (12) |
The high pressure (>2.5 MPa, depending on the gasifier type), high temperature (>1000 °C), spent air produced from the combustor can be used to drive a gas turbine–steam turbine combined cycle system to generate electricity for parasitic energy consumptions. In yet another configuration, a fraction or all of the reduced particles from the reducer can bypass the oxidizer and be introduced directly to the combustor if more heat or electricity is desired. Hence, both chemical-looping reforming and chemical-looping combustion concepts are applied in the SCL system, rendering it a versatile technology for H2 and electricity co-production.
The SCL process has been tested at Ohio State University (OSU) in a 2.5 kWth bench scale moving bed unit for a combined operating time of >100 h.154 Current testing results indicate) >99.9% syngas conversion in the reducer and >99.95% purity hydrogen stream from the oxidizer. Nearly full conversion of gaseous hydrocarbons such as CH4 was also obtained. A 25 kWth SCL demonstration unit is being constructed at OSU. The process analysis based on the bench scale testing results indicated that the overall efficiency for the SCL process can exceed 64% (HHV) with 100% CO2 capture. For comparison, the efficiency of a traditional coal-to-hydrogen process with 90% CO2 capture is estimated to be 57% (HHV).155
Besides serving as a stand alone hydrogen/electricity producer, the SCL process can be integrated into other processes to improve the overall energy conversion scheme. Fig. 12 exemplifies the integration of the SCL process to the state-of-the-art Coal-to-Liquids (CTL) process.156 In this configuration, the SCL system converts the C1–C4 products from the Fischer–Tropsch (FT) reactor into H2 and recycles it to the F–T reactor as feedstock, resulting in a 10% increase in the liquid fuel yield and a 19% reduction in CO2 emissions.157
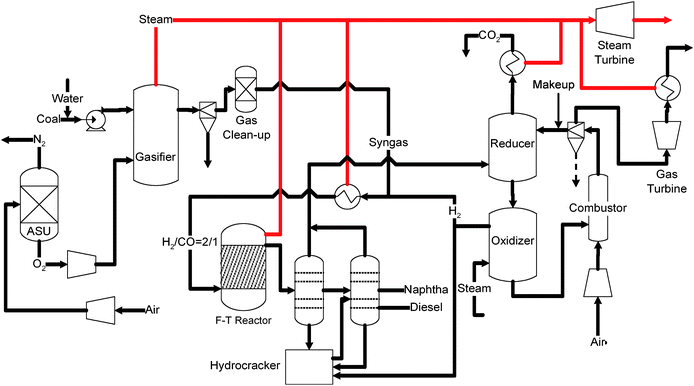 | ||
| Fig. 12 Syngas Chemical Looping enhanced Coal-to-Liquids (SCL–CTL) process. | ||
Oxygen carriers other than iron oxide such as NiO were also explored for hydrogen generation from syngas. The experiments carried out in a 20 mm I.D. fixed bed reactor, however, indicated that Fe is a more favorable choice than Ni.158,159 Svodoba et al. (2007,2008)160,161 also examined, using thermodynamic principles, the feasibility of using Fe, Mn, Ni, Cr, and Co based particles for hydrogen production. They concluded that Fe–Fe3O4 is more suitable for chemical looping gasification compared to other particles; however, they further stated that Fe3O4 is more difficult to reduce based on a fluidized bed design. Xiang et al. (2007) performed ASPEN Plus® simulation on an iron-based looping system for hydrogen generation.162 In their system, reduced iron oxide is only regenerated to Fe3O4 rather than Fe2O3. As a result, a significant amount of syngas will leave the reducer unconverted. Based on the simulation results, the system has an energy conversion efficiency as high as 58.33% (LHV).
Calcium looping process (CLP). Fig. 13 shows the schematic integration of the calcium looping process in a typical coal gasification system for the production of hydrogen.52,156,163,164
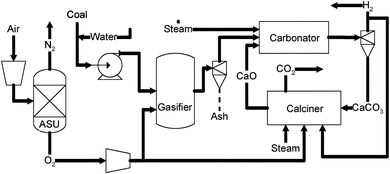 | ||
| Fig. 13 Schematic flow diagram of the calcium looping process. | ||
As shown in Fig. 13, the calcium looping process comprises two reactors: the carbonation reactor (carbonator), which produces high purity hydrogen while removing contaminants, and the calciner, where the calcium sorbent is regenerated and a concentrated CO2 stream is produced. The carbonator is operated at 550–650 °C and 2–3 MPa (20–30 atm). In the carbonator, the CO2 generated by the WGS reaction is simultaneously removed by a CaO sorbent. The mesoporous, precipitated calcium carbonate (PCC–CaO) sorbent has much higher reactivity and CO2 capture capacity (40–36 wt % for 50th–100th cycles) when compared to most of the high temperature sorbents reported in the literature. Moreover, it is capable of capturing the sulfur and halides in the raw syngas stream. Hence, the high performance PCC–CaO sorbent captures the pollutants in the syngas while driving the thermodynamic equilibrium of the WGS reaction towards the formation of hydrogen until 100% of the CO is consumed. As a result, high purity hydrogen with very low concentration of H2S, COS, and HCl can be produced with drastically reduced steam consumption (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = ∼1
CO = ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reactions occurring in the carbonator are as follows:
1). The reactions occurring in the carbonator are as follows:
| CO + H2O → H2 + CO2 | (13) |
| CaO + CO2 → CaCO3 | (14) |
| CaO + H2S → CaS + H2O | (15) |
| CaO + COS → CaS + CO2 | (16) |
| CaO + 2HCl → CaCl2 + H2O | (17) |
The spent sorbent, consisting mainly of CaCO3, is then recycled to the calciner, where heat is provided to regenerate the carbonated sorbent. The calciner operates at 800–1000 °C and ambient pressure.
| CaCO3 → CaO + CO2 | (18) |
A mixture of CO2 and steam will be produced from the calciner. After condensing the steam, CO2 can be compressed and transported for sequestration.
Hence, this technology provides an efficient “one box” mode of operation for the production of high purity hydrogen with CO2, sulfur and chloride capture that integrates the WGSR, CO2 capture, sulfur removal and hydrogen separation in one consolidated unit. High purity hydrogen (>99.9%) was produced from a lab scale testing unit. ASPEN® Plus simulations showed that the overall efficiency of the process for hydrogen production is 63% (HHV).156 The large amount of heat required for the calcination reactor and the sorbent reactivity after regeneration under an elevated temperature represents a major challenge to the CLP.
Thermal swing sorption-enhanced reaction (TSSER) process. The TSSER process also uses CO2 sorbent to enhance the WGS reaction and H2 production. The differences between the TSSER process and the CLP lie in the sorbent properties, reactor system design, and operating conditions. The sorbents used in the TSSER process such as K2CO3 promoted hydrotalcite and Na2O promoted alumina cannot capture pollutants from syngas; therefore, the contaminants in the syngas need to be removed before entering the sorbent bed. Moreover, the TSSER is composed of multiple (fixed) sorbent beds operating in a sequential manner, which is similar to the operations of the PSA system. The TSSER process is currently under the lab scale testing. Fuel cell grade hydrogen has been produced from a 17.3 mm diameter fixed bed reactor.165,166 The potential challenges to the TSSER process include a relatively low CO2 capture capacity of the sorbent (<4.4 wt %)166 and constant temperature and pressure swings in fixed beds under relatively high temperature (300–550 C).
Sorbent regeneration represents a crucial step to Type B chemical looping processes. Since a significant amount of heat is required for sorbent regeneration, an optimized energy integration scheme is necessary in order to achieve high energy conversion efficiency. Moreover, regeneration conditions can have notable effects on the sorbent recyclability.
4.3 Direct coal chemical looping processes
Both the membrane and the syngas chemical looping approaches discussed in the previous sections enhance the conventional coal gasification processes by integrating the WGS and CO2 removal steps into the looping scheme. The advanced coal gasification processes discussed in this section incorporate, not only the WGS and CO2 removal steps, but also the coal gasification step. As a result, the coal conversion process is further simplified.Chemical looping combustion of coal. Compared to syngas, coal is more difficult to react. The contaminants in coal that may react with oxygen carrier particles make a direct oxidation of coal an even more challenging task. Zhao et al. (2008) proposed to use NiO based oxygen carrier particles (NiO 60% by weight) obtained from sol–gel technique to convert coal char.167 A TGA experiment indicted noticeable coal char–NiO conversion over a period of 120 min As noted, the major challenge associated with NiO based looping processes is the high oxygen carrier cost, which is especially the case when the elaborate sol–gel technique is used for synthesizing the particle. Further, the slow reaction kinetics between NiO and char indicates the necessity for a char gasification promoter.
Yang et al. (2007) proposed to use Fe2O3 as the oxygen carrier particles to covert coal.168 Fixed bed studies were performed which indicate that Fe2O3 can be converted to Fe3O4 using coal volatiles and gasified coal gas (CO and H2). However, reduction from Fe2O3 to Fe3O4 only utilizes 11.13% of the maximum oxygen carrying capacity of Fe2O3. Scott et al. (2006) also utilized Fe2O3 as an oxygen carrier particle169 to convert char in a small fluidized bed. In their experiments, char was fed into a small fluidized bed. With the presence of CO2, char was gasified and then reacted with Fe2O3. It was found that Fe2O3 can only be reduced to Fe3O4 in the fluidized bed due to thermodynamic limitations. Cao et al. (2006) proposed to use CuO as an oxygen carrier particle to combust coal170,171 in a circulating fluidized bed with only the available data obtained from TGA. It is noted that the low melting point of Cu/CuO can be a serious issue in its applications. Studies carried out by others indicate that copper based particles will deactivate beyond 800 °C.172 The low operating temperature will lead to significantly reduced energy conversion efficiency.
The direct coal CLC processes are at the early stage of development and further studies in particle development, process design, and analysis are necessary in order to assess the technical feasibility and the commercial readiness for these processes.
Chemical looping gasification of coal—coal direct chemical looping process. The coal direct chemical looping (CDCL) process, illustrated in Fig. 14, is capable of converting coal into hydrogen and/or electricity at any relative proportions.137,138,156,173 In the CDCL process, composite Fe2O3 particles are introduced into the reducer to react with pulverized coal. The reducer is a two-stage moving bed reactor, which provides a desired gas–solid contacting pattern. In the reducer, coal is gasified in-situ and reacted with Fe2O3 particles. Thus, a mixture of Fe and FeO is produced along with a flue gas stream composed of CO2, H2O, and contaminants such as H2S and elemental mercury. After condensing out the steam, the flue gas can be compressed and sequestrated. A portion of the reduced Fe/FeO particle from the reducer will enter the oxidizer to react with steam to form hydrogen. The resulting Fe3O4 exiting the oxidizer along with the remaining portion of the reduced Fe/FeO particle will be combusted with air in the entrained flow combustor. The combustor conveys the particle back to the reducer pneumatically while regenerating the particle to its original oxidized form. Part of the heat released in the combustor will be carried to the reducer by the hot particles to compensate the endothermic heat required in the reducer. The remaining heat released in the combustor heats up the exhaust gas, which can be used for steam or electricity generation.
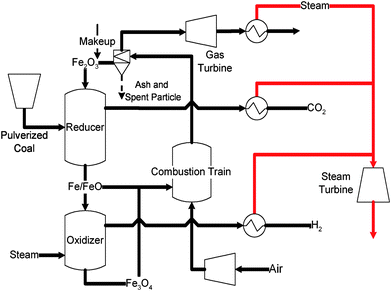 | ||
| Fig. 14 Schematic diagram of Coal-Direct Chemical Looping process. | ||
The CDCL process testing has been carried out in the 2.5 kWth bench scale moving bed unit at OSU. Different feedstock such as coal volatiles (simulated), lignite coal char, bituminous coal char, and anthracite coal have been tested. Coal/coal char conversion of as high as 95.5% has been obtained. The CO2 concentration in the exhaust stream was >97% (dry basis) in all cases. Moreover, the reactivity of the particles was maintained after three redox cycles in which coal was used as the reducing agent. ASPEN Plus® simulation showed that the energy conversion efficiency of the CDCL process was higher 80% (HHV) for hydrogen production and over 50% for electricity generation with zero carbon emissions.156,174
HyPr-Ring process. Similar to the CO2 Acceptor process developed by the Consolidation Coal Company in the 1960’s, the HyPr-Ring process developed in Japan involves coal gasification using pure oxygen and steam.175–178Fig. 15 illustrates the HyPr-Ring process. In this process, coal is fed to the gasifier along with calcium oxide, steam and oxygen. The presence of excessive steam and the in-situ CO2 removal by calcium oxide drives the equilibrium in the gasifier towards the formation of H2. As a result, a product gas stream of up to 90% H2 mixed with methane, other hydrocarbons, and sulfur and nitrogen based contaminants is generated.178 The solids from the gasifier consist mostly of saturated CaO sorbents (CaCO3) and unconverted carbon that is to be introduced to a regenerator along with oxygen. The heat generated by combusting the unreacted carbon by oxygen allows the calcination reaction to be carried out for CaO regeneration while producing high purity CO2 for sequestration. The challenges for the HyPr-Ring process include the deactivation of CaO in the presence of coal ash176 and relatively low purity hydrogen product from the gasifier. The HyPr-Ring process is currently under demonstration in a pilot scale unit with a coal processing rate of 3.5 kg h−1. Process analysis showed that a 77% cold gas efficiency (HHV) can be achieved when CO2 compression was not taken into account.175
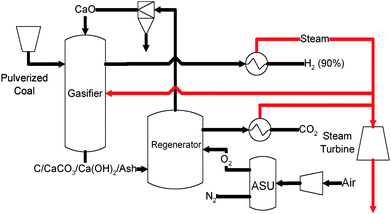 | ||
| Fig. 15 Schematic diagram of HyPr-Ring process.175 | ||
Different from either Type A or Type B chemical looping systems, the GE Fuel–Flexible Process179,180 and the ALSTOM Hybrid Combustion–Gasification Chemical Looping process181 employ two separate looping particles, i.e., an oxygen carrier and a CO2 sorbent, to carry out the coal conversion. Thus, there are two separate looping schemes in each of these two looping processes. In these processes, the CO2 sorbent is used to enhance the hydrogen generation while the oxygen carrier is either used for indirect combustion of fuel to provide the heat needed for spent sorbent reactivation or used for coal gasification. These processes can convert coal into a variety of products with in-situ carbon dioxide capture. The mixing between the oxygen carrier particles and sorbent particles as well as significantly large solid handling requirements render these processes more difficult to operate as compared to chemical looping processes that involve single chemical reaction loop.
5. Concluding remarks
Coal will remain to be an important energy source well into the 21st century. With a strong demand for an affordable energy supply which is compounded by the urgent needs for CO2 emission control, the clean and efficient utilization of coal represents both a major opportunity and challenge to current global R&D efforts in this area.The coal conversion processes of the future prospect are plotted in Fig. 16 along with the current or demonstrated processes for electricity and/or H2 production. These efficiencies are given considering a CO2 controlled environment. The processes considered in the figure include sub-critical and ultra supercritical PC processes retrofit with either MEA or chilled ammonia system for CO2 capture, coal gasification processes using the SELEXOL system for CO2 capture, the H2-selective membrane based gasification process, syngas chemical looping processes, and the coal direct chemical looping process (CDCL).
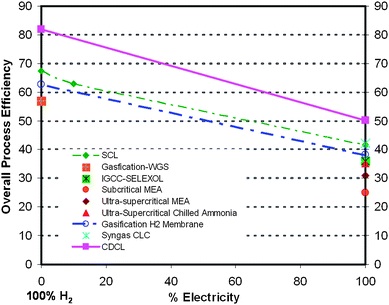 | ||
| Fig. 16 Efficiency comparisons among various coal conversion technologies with >90% CO2 capture† | ||
It is seen that in terms of electricity or H2 generation, the efficiencies for syngas chemical looping processes and H2-selective membrane process are comparable and could be considered as near term retrofit technology for current coal gasification processes. Of particular noteworthiness is the CDCL process, which shows considerably higher energy conversion efficiency than all the other processes considered. The direct coal chemical looping processes can emerge as attractive clean coal conversion systems for the intermediate term.
Coal is used as the feedstock in the current discussion. The application of the technologies discussed in this paper, however, is extendable to other carbonaceous feedstock such as petroleum coke, biomass, and refuse derived fuel. From the small scale testing results, it is encouraging that the novel coal conversion process based on chemical looping and membrane concepts may become commercially viable in the foreseeable future. And the direct coal chemical looping processes may become a reality.
Acknowledgements
This work has been supported by the Ohio Coal Development Office of the Ohio Air Quality Development Authority, U. S. Department of Energy, Ohio State University and an industrial consortium.References
- World Meteorological Organization (WMO), ScienceDaily, 2006, November 4, http://www.sciencedaily.com/releases/2006/11/061104084951.htm, Accessed 1 May, 2008 Search PubMed.
- National Oceanic and Atmospheric Administration (NOAA), After Two Large Annual Gains, Rate of Atmospheric CO2 Increase Returns to Average, http://www.publicaffairs.noaa.gov/releases2005/mar05/noaa05-035.html, Accessed 1 May, 2008 Search PubMed.
- J. Hansen and M. Sato, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16109–16114 CrossRef CAS.
- S. Jacobsson and A. Johnson, Energy Policy, 2000, 28, 625–640 CrossRef.
- T. McVeigh, D. Burtraw, J. Darmstadter and K. Palmer, Sol. Energy, 2000, 68, 237–255 CrossRef.
- R. C. Ewing, MRS Bull., 2008, 33, 338–340 CAS.
- Energy Information Administration (EIA), in International Energy Outlook 2007. EIA, U.S. Department of Energy, Washington DC, 2007 Search PubMed.
- Intergovernmental Panel on Climate Change (IPCC), in Climate Change 2007: Synthesis Report. IPCC, Valencia, 2007 Search PubMed.
- W. Ying and W. Zhu, International Herald Tribune, 2007, http://www.iht.com/articles/2007/04/16/business/sxasia.php, Accessed 29 April, 2008 Search PubMed.
- Britannica, Coal Mining, http://www.britannica.com/eb/article-9110682/coal-mining, Accessed 28 April, 2008 Search PubMed.
- Britannica, Coal, http://www.britannica.com/eb/article-9110442/coal, Accessed 28 April, 2008 Search PubMed.
- T. S. Ashton, in The Industrial Revolution, 1760–1830, Oxford University Press, New York, 1997 Search PubMed.
- R. Church, in The History of the British Coal Industry, 1830–1913, Clarendon Press, Oxford, 1986, vol. 3 Search PubMed.
- The Babcock & Wilcox Company, in Steam, its generation and use, ed. S. C. Stultz and J. B. Kitto, Babcock & Wilcox, Barberton, Ohio, 40th edn, 1992 Search PubMed.
- B. G. Miller, Coal Energy Systems, Elsevier, Amsterdam, Boston, 2005 Search PubMed.
- S. Yeh and E. S. Rubin, Energy, 2007, 32, 1996–2005 CrossRef CAS.
- Massachusetts Institute of Technology (MIT), in The Future of Coal: Options for a Carbon-Constrained World, MIT, Boston, 2007 Search PubMed.
- J. Bugge, S. Kjaer and R. Blum, Energy, 2006, 31, 1437–1445 CrossRef CAS.
- J. Stringer and L. A. Ruth, Mater. High Temp., 2003, 20, 233–239 Search PubMed.
- M. J. Bradley and B. M. Jones, AMBIO: J. Human Environ., 2002, 31, 141–149 Search PubMed.
- S. Banales-Lopez and V. Norberg-Bohm, Energy Policy, 2002, 30, 1173–1180 CrossRef.
- U.S. Department of Energy (USDOE), Fluidized Bed Technology - Overview, http://www.fossil.energy.gov/programs/powersystems/combustion/fluidizedbed_overview.html, Accessed 27 April, 2008 Search PubMed.
- J. M. Rockey and R. E. Weinstein, Proceedings of the 15th International Conference on Fluidized Bed Combustion, Savannah, Georgia, USA, 1999 Search PubMed.
- U.S. Department of Energy (USDOE), in The JEA large-scale CFB combustion demonstration project: A report on a project conducted jointly under a cooperative agreement between the U.S. Department of Energy and JEA, U.S. Department of Energy, Washington DC, 2003 Search PubMed.
- J. Watson, Proceedings of the British Insitute of Energy Economics (BIEE) Academic Conference, St. John's College, Oxford, UK, 2005 Search PubMed.
- A. J. Minchener, Proc. Inst. Mech. Eng. A-J Power, 2003, 217, 9–18 Search PubMed.
- M. C. Bohm, MSc Thesis, Massachusetts Institute of Technology, 2006 Search PubMed.
- M. C. Woods, P. J. Capicotto, J. L. Haslbeck, N. J. Kuehn, M. Matuszewski, L. L. Pinkerton, M. D. Rutkowski, R. L. Schoff and V. Vaysman, Cost and Performance Baseline for Fossil Energy Plants, DOE Report, DOE/NETL-2007/1281, NETL, US Department of Energy, 2007 Search PubMed.
- M. Ramezan, T. J. Skone, N. Y. Nasakala and G. N. Liljedahl, Carbon Dioxide Capture from Existing Coal-Fired Power Plants, DOE Report, DOE/NETL-401/110907, NETL, US Department of Energy, 2007 Search PubMed.
- Y. Lu, S. Chen, M. Rostam-Abadi, R. Varagani, F. Chatel-Pelage and A. C. Bose, in Proceedings of the 22nd Annual International Pittsburgh Coal Conference, Pittsburgh, PA, USA 2005 Search PubMed.
- E. S. Rubin, C. Chen and A. B. Rao, Energy Policy, 2007, 35, 4444–4454 CrossRef.
- H. Hutchinson, Mech. Eng., 2002, 124, 41–45 Search PubMed.
- H. Gupta, T. J. Thomas, A. H. A. Park, M. V. Iyer, P. Gupta, R. Agnihotri, R. A. Jadhav, H. W. Walker, L. K. Weavers, T. Butalia and L.-S. Fan, Ind. Eng. Chem. Res., 2007, 46, 5051–5060 CrossRef CAS.
- L. S. Fan, A. Ghosh-Dastidar and S. Mahuli, US Pat., 5 779 464, 1998 Search PubMed.
- L.-S. Fan and R. A. Jadhav, AIChE J., 2002, 48, 2115–2123 CrossRef CAS.
- H. Gupta, S. A. Benson, L.-S. Fan, J. D. Laumb, E. S. Olson, C. R. Crocker, R. K. Sharma, R. Z. Knutson, A. S. M. Rokanuzzaman and J. E. Tibbets, Ind. Eng. Chem. Res., 2004, 43, 5820–5827 CrossRef CAS.
- P. Taerakul, P. Sun, D. W. Golightly, H. W. Walker, L. K. Weavers, B. Zand, T. Butalia, T. J. Thomas, H. Gupta and L.-S. Fan, Fuel, 2007, 86, 541–553 CrossRef CAS.
- E. A. Bryant, in Climate Process & Change, Cambridge University Press, Cambridge, New York, 1997 Search PubMed.
- J. N. Armor, Catal. Lett., 2007, 114, 115–121 CrossRef CAS.
- M. R. M. Abu-Zahra, J. P. M. Niederer, P. H. M. Feron and G. F. Versteeg, Int. J. Greenhouse Gas Control, 2007, 1, 135–142 CrossRef CAS.
- M. R. M. Abu-Zahra, L. H. J. Schneiders, J. P. M. Niederer, P. H. M. Feron and G. F. Versteeg, Int. J. Greenhouse Gas Control, 2007, 1, 37–46 CrossRef CAS.
- D. Singh, E. Croiset, P. L. Douglas and M. A. Douglas, Energy Convers. Manage., 2003, 44, 3073–3091 CrossRef CAS.
- A. B. Rao and E. S. Rubin, Environ. Sci. Technol., 2002, 36, 4467–4475 CrossRef CAS.
- T. Mattisson, A. Lyngfelt and P. Cho, Fuel, 2001, 80, 1953–1962 CrossRef CAS.
- F. Châtel-Pélage, R. Varagani, P. Pranda, N. Perrin, H. Farzan, S. J. Vecci, Y. Lu, S. Chen, M. Rostam-Abadi and A. C. Bose, Proceedings of the Second International Conference on Clean Coal Technologies for our Future, Sardinia, Italy, 2005 Search PubMed.
- Chilling News for Carbon Capture, http://www.modernpowersystems.com/story.asp%3FstoryCode%3D2045485, Accessed 5 May, 2008 Search PubMed.
- Chilled-ammonia Post Combustion CO2 Capture System—Laboratory and Economic Evaluation Results.EPRI, Palo Alto, CA: 2006 1012797.
- Electric Power Research Institute, in EPRI J., Spring, 2007 Search PubMed.
- M. A. Gary, Carbon Capture Demonstration Projects: AEP's Perspective, Western Governors' Association Workshop, http://www.westgov.org/wga/meetings/coal07/GRAY.ppt%23256, Accessed 4 May, 2008 Search PubMed.
- C. M. White, B. R. Strazisar, E. J. Granite, J. S. Hoffman and H. W. Pennline, J. Air Waste Manage., 2003, 53, 645–715 Search PubMed.
- D. Aaron and C. Tsouris, Sep. Sci. Technol., 2005, 40, 321–348 CrossRef CAS.
- M. V. Iyer, H. Gupta, B. B. Sakadjian and L.-S. Fan, Ind. Eng. Chem. Res., 2004, 43, 3939–3947 CrossRef CAS.
- L.-S. Fan and M. Iyer, TCE Today, October 2006, 36–38 Search PubMed.
- B. B. Sakadjian, W. K. Wang, D. M. Wong, M. V. Iyer, S. Ramkumar, S. Li, L.-S. Fan and R. M. Statnick, Proceedings of the 33rd International Conference of Coal Utilization and Fuel Systems, Clear Water, Florida, USA, 1–5 June, 2008, in press Search PubMed.
- D. Y. Lu, R. W. Hughes and E. J. Anthony, Proceedings of the 9th International Conference on Circulating Fluidized Beds, Hamburg, Germany, 2008 Search PubMed.
- J. C. Abanades, G. Grasa, M. Alonso, N. Rodriguez, E. J. Anthony and L. M. Romeo, Environ. Sci. Technol., 2007, 41, 5523–5527 CrossRef CAS.
- E. J. Anthony, Ind. Eng. Chem. Res., 2008, 47, 1747–1754 CrossRef CAS.
- L. I. Eide, M. Anheden, A. Lyngfelt, C. Abanades, M. Younes, D. Clodic, A. A. Bill, P. H. M. Feron, A. Rojey and F. Giroudiere, Oil & Gas Science and Technology-Revue De L Institut Francais Du Petrole, 2005, 60, 497–508 Search PubMed.
- T. Shimizu, T. Hirama, H. Hosoda, K. Kitano, M. Inagaki and K. Tejima, Chem. Eng. Res. Des., 1999, 77, 62–68 CrossRef CAS.
- A. Seltzer, Z. Fan and H. Hack, Proceedings of the 7th Annual COAL-GEN Conference, Milwankee, WI, USA, 2007, http://www.fosterwheeler%20canadaltd.com/publications/tech_papers/files/TP_CCS_07_03.pdf, Accessed 5 May, 2008 Search PubMed.
- B. J. P. Buhre, L. K. Elliott, C. D. Sheng, R. P. Gupta and T. F. Wall, Prog. Energy Combust. Sci., 2005, 31, 283–307 CrossRef CAS.
- C. Higman, Gasification, Gulf Professional, Boston, Massachusetts, 2nd edn, 2008 Search PubMed.
- G. J. Stiegel and M. Ramezan, Int. J. Coal Geol., 2006, 65, 173–190 CAS.
- O. Shinada, A. Yamada and Y. Koyama, Energy Convers. Manage., 2002, 43, 1221–1233 CrossRef CAS.
- A. L. Kohl and R. Nielsen, Gas purification, Gulf Publishing Company, Houston, Texas, 5th edn, 1997 Search PubMed.
- M. E. Dry, Appl. Catal., A, 1996, 138, 319–344 CrossRef CAS.
- M. E. Dry, Abstr. Pap. Am. Chem. Soc., 2000, 219, U254–U254.
- W. P. Ma, Y. L. Zhao, Y. W. Li, Y. Y. Xu and J. L. Zhou, React. Kinet. Catal. Lett., 1999, 66, 217–223 CrossRef CAS.
- X. Li, S. X. Hu, L. J. Jin and H. Q. Hu, Energy Fuels, 2008, 22, 1126–1129 CrossRef CAS.
- A. G. Comolli, T. L. K. Lee, G. A. Popper and P. Z. Zhou, Fuel Process. Technol., 1999, 59, 207–215 CrossRef CAS.
- Q. Y. Sun, J. J. Fletcher, Y. Z. Zhang and X. K. Ren, Energy Fuels, 2005, 19, 1160–1164 CrossRef CAS.
- D. Lewandowski and D. Gray, Presented at the 2001 Gasification Technologies Conference, 2001http://www.gasification.org/Docs/Conferences/2001/GTC01019.pdf, Accessed 5 May, 2008 Search PubMed.
- M. Kanniche and C. Bouallou, Appl. Therm. Eng., 2007, 27, 2693–2702 CrossRef.
- K. Damen, M. van Troost, A. Faaij and W. Turkenburg, Prog. Energy Combust. Sci., 2006, 32, 215–246 CrossRef CAS.
- National Energy Technology Laboratory, Carbon Sequestration: CO2 Capture, http://www.netl.doe.gov/technologies/carbon_seq/core_rd/co2capture.html, Accessed 4 May, 2008 Search PubMed.
- R. Hotchkiss, Proc. Inst. Mech. Eng. A-J Power, 2003, 217, 27–33 Search PubMed.
- P. N. Dyer, R. E. Richards, S. L. Russek and D. M. Taylor, Solid State Ionics, 2000, 134, 21–33 CrossRef CAS.
- R. W. Baker, Membrane separation systems recent developments and future directions, Noyes Data Corp., Park Ridge, NJ, 1991 Search PubMed.
- N. K. Park, D. H. Lee, J. H. Jun, J. D. Lee, S. O. Ryu, T. J. Lee, J. C. Kim and C. H. Chang, Fuel, 2006, 85, 227–234 CrossRef CAS.
- S. Poulston, E. J. Granite, H. W. Pennline, C. R. Myers, D. P. Stanko, H. Hamilton, L. Rowsell, A. W. J. Smith, T. Ilkenhans and W. Chu, Fuel, 2007, 86, 2201–2203 CrossRef CAS.
- J. Smid, S. S. Hsiau, C. Y. Peng and H. T. Lee, Filtr. Sep., 2006, 43, 21–24 CrossRef CAS.
- X. Xu, Y. Xiao and C. Qiao, Energy Fuels, 2007, 21, 1688–1694 CrossRef CAS.
- H. M. Yan and V. Rudolph, Chem. Eng. Commun., 2000, 183, 1–38 CrossRef CAS.
- W. F. Elseviers, T. VanMierlo, M. J. F. VandeVoorde and H. Verelst, Fuel, 1996, 75, 1449–1456 CrossRef CAS.
- B. Wang, R. Yan, D. H. Lee, D. T. Liang, Y. Zheng, H. Zhao and C. Zheng, Energy Fuels, 2008, 22, 1012–1020 CrossRef CAS.
- L. Zhou, S. Y. Hu, Y. R. Li and Q. H. Zhou, Chem. Eng. J., 2008, 136, 31–40 CrossRef CAS.
- Y. H. Zhu and H. C. Frey, J. Air Waste Manage., 2006, 56, 1649–1661 Search PubMed.
- N. W. Ockwig and T. M. Nenoff, Chem. Rev., 2007, 107, 4078–4110 CrossRef CAS.
- J. Zou and and W. S. W. Ho, J. Membr. Sci., 2006, 286, 310–321 CrossRef.
- A. E. Ayturk, I. P. Mardilovich, E. E. Engwall and Y. H. Ma, J. Membr. Sci., 2006, 285, 385–394 CrossRef.
- K. S. Rothenberger, A. V. Cugini, B. H. Howard, R. P. Killmeyer, M. V. Ciocco, B. D. Morreale, R. M. Enick, F. Bustamante, I. P. Mardilovich and Y. H. Ma, J. Membr. Sci., 2004, 244, 55–68 CrossRef CAS.
- O. Iyoha, R. Enick, R. Killmeyer, B. Howard, M. Ciocco and B. Morreale, J. Membr. Sci., 2007, 306, 103–115 CAS.
- S. Hara, K. Sakaki, N. Itoh, H. M. Kimura, K. Asami and A. Inoue, J. Membr. Sci., 2000, 164, 289–294 CrossRef CAS.
- D. S. Sholl and Y. H. Ma, MRS Bull., 2006, 31, 770–773 CAS.
- K. Jordal, R. Bredesen, H. M. Kvamsdal and O. Bolland, Energy, 2004, 29, 1269–1278 CrossRef CAS.
- B. A. McCool and Y. S. Lin, J. Mater. Sci., 2001, 36, 3221–3227 CrossRef CAS.
- P. Kamakoti, B. D. Morreale, M. V. Ciocco, B. H. Howard, R. P. Killmeyer, A. V. Cugini and D. S. Sholl, Science, 2005, 307, 569–573 CrossRef CAS.
- H. Yukawa, A. Teshima, D. Yamashita, S. Ito, S. Yamaguchi and M. Morinaga, J. Alloys Compd., 2002, 337, 264–268 CrossRef CAS.
- S. Hara, N. Hatakeyama, N. Itoh, H. M. Kimura and A. Inoue, J. Membr. Sci., 2003, 211, 149–156 CrossRef CAS.
- H. Verweij, J. Mater. Sci., 2003, 38, 4677–4695 CrossRef CAS.
- H. Verweij, Y. S. Lin and J. H. Dong, MRS Bull., 2006, 31, 756–764 CAS.
- M. C. Mitchell, M. Gallo and T. M. Nenoff, J. Chem. Phys., 2004, 121, 1910–1916 CrossRef CAS.
- Y. Yoshino, T. Suzuki, B. N. Nair, H. Taguchi and N. Itoh, J. Membr. Sci., 2005, 267, 8–17 CrossRef CAS.
- R. Bredesen, K. Jordal and O. Bolland, Chem. Eng. Process., 2004, 43, 1129–1158 CrossRef CAS.
- M. P. Bernal, J. Coronas, M. Menendez and J. Santamaria, AIChE J., 2004, 50, 127–135 CrossRef CAS.
- S. G. Li, J. G. Martinek, J. L. Falconer, R. D. Noble and T. Q. Gardner, Ind. Eng. Chem. Res., 2005, 44, 3220–3228 CrossRef CAS.
- S. Sircar, M. Rao and C. Thaeron, Sep. Sci. Technol., 1999, 34, 2081 CrossRef CAS.
- S. Sircar, W. E. Waldron, M. B. Rao and M. Anand, Sep. Purif. Technol., 1999, 17, 11–20 CrossRef CAS.
- F. Kapteijn, W. J. W. Bakker, J. Vandegraaf, G. Zheng, J. Poppe and J. A. Moulijn, Catal. Today, 1995, 25, 213–218 CrossRef CAS.
- H. Q. Lin and B. D. Freeman, Macromolecules, 2005, 38, 8394–8407 CrossRef CAS.
- H. Q. Lin, T. Kai, B. D. Freeman, S. Kalakkunnath and D. S. Kalika, Macromolecules, 2005, 38, 8381–8393 CrossRef CAS.
- T. Yamaguchi, C. A. Koval, R. D. Noble and C. N. Bowman, Chem. Eng. Sci., 1996, 51, 4781–4789 CrossRef CAS.
- H. Matsuyama, M. Teramoto, H. Sakakura and K. Iwai, J. Membr. Sci., 1996, 117, 251–260 CrossRef CAS.
- S. P. Kaldis, G. Skodras and G. P. Sakellaropoulos, Fuel Process. Technol., 2004, 85, 337–346 CrossRef CAS.
- M. Amelio, P. Morrone, F. Galucci and A. Basile, Energy Convers. Manage., 2007, 48, 2680–2693 CrossRef CAS.
- D. Grainger and M.-B. Hägg, Fuel, 2008, 87, 14–24 CrossRef CAS.
- M. V. Ciocco, O. Iyoha, R. M. Enick and R. P. Killmeyer, High-Temperature Water–Gas Shift Membrane Reactor Study, 2007 Search PubMed.
- M. C. Carbo, D. Jansen, W. G. Haije and A. H. M. Verkooijen, M. C. Carbo, D. Jansen, W. G. Haije and A. H. M. Verkooijen, Proceedings of Fifth Annual Conference on Carbon Capture and Sequestration, Alexandria VA, USA, 2006 Search PubMed.
- P. Chiesa, T. G. Kreutz and G. G. Lozza, J. Eng. Gas Turbines Power, 2007, 129, 123–134 CrossRef CAS.
- J. Huang and W. S. W. Ho, J. China Inst. Chem. Eng., 2008, 39, 129–136 Search PubMed.
- D. Shain, O. Estela, A. Mike, L. Francis and R. Mike, A Novel Membrane Reactor for Direct Hydrogen Production From Coal, United States, 2006 Search PubMed.
- US Pat., 1 078 686, 1913 Search PubMed.
- S. Hurst, J. Am. Oil Chem. Soc., 1939, 16, 29–36 CrossRef CAS.
- US Pat., 2 665 971, 1954 Search PubMed.
- US Pat., 2 665 972, 1954 Search PubMed.
- M. Anheden and G. Svedberg, Energy Convers. Manage., 1998, 39, 1967–1980 CrossRef CAS.
- J. Dewulf, H. Van Langenhove, B. Muys, S. Bruers, B. R. Bakshi, G. F. Grubb, D. M. Paulus and E. Sciubba, Environ. Sci. Technol., 2008, 42, 2221–2232 CrossRef CAS.
- M. Ishida, D. Zheng and T. Akehata, Energy, 1987, 12, 147–154 CrossRef CAS.
- H. G. Jin and M. Ishida, Fuel, 2004, 83, 2411–2417 CrossRef CAS.
- M. Johansson, T. Mattisson and A. Lyngfelt, Ind. Eng. Chem. Res., 2006, 45, 5911–5919 CrossRef CAS.
- P. Gupta, L. G. Velazquez-Vargas and L. S. Fan, Energy Fuels, 2007, 21, 2900–2908 CrossRef CAS.
- L. S. Fan and F. X. Li, Phys. World, 2007, 20, 37–41 Search PubMed.
- H. Leion, T. Mattisson and A. Lyngfelt, Fuel, 2007, 86, 1947–1958 CrossRef CAS.
- W. Xiang, S. Wang and T. T. Di, Energy Fuels, 2008, 22, 961–966 CrossRef CAS.
- L. F. de Diego, F. Garcia-Labiano, J. Adanez, P. Gayan, A. Abad, B. M. Corbella and J. M. Palacios, Fuel, 2004, 83, 1749–1757 CrossRef CAS.
- R. Gabbrielli and R. Singh, J. Eng. Gas Turbines Power, 2003, 125, 940–946 CrossRef CAS.
- WO Pat., 2 007 082 089, 2007 Search PubMed.
- US Pat., 11 010 648, 2004 Search PubMed.
- L. G. Velazquez-Vargas, T. Thomas, P. Gupta and L.-S. Fan, Proceedings of the 2004 AIChE Annual Technical Meeting,Austin, Texas, USA 2004 Search PubMed.
- A. Abad, J. Adanez, F. Garcia-Labiano, L. F. de Diego, P. Gayan and J. Celaya, Chem. Eng. Sci., 2007, 62, 533–549 CrossRef CAS.
- R. Siriwardane, J. Poston, K. Chaudhari, A. Zinn, T. Simonyi and C. Robinson, Energy Fuels, 2007, 21, 1582–1591 CrossRef CAS.
- M. Ryden, A. Lyngfelt, T. Mattisson, D. Chen, A. Holmen and E. Bjorgum, Int. J. Greenhouse Gas Control, 2008, 21–36 CrossRef CAS.
- F. Garcia-Labiano, J. Adanez, L. F. de Diego, P. Gayan and A. Abad, Energy Fuels, 2006, 20, 26–33 CrossRef CAS.
- E. Johansson, T. Mattisson, A. Lyngfelt and H. Thunman, Fuel, 2006, 85, 1428–1438 CrossRef CAS.
- E. Johansson, T. Mattisson, A. Lyngfelt and H. Thunman, Chem. Eng. Res. Des., 2006, 84, 819–827 CrossRef CAS.
- T. Mattisson, F. García-Labiano, B. Kronberger, A. Lyngfelt, J. Adánez and H. Hofbauer, Int. J. Greenhouse Gas Control, 2007, 1, 158–169 CAS.
- A. Abad, T. Mattisson, A. Lyngfelt and M. Johansson, Fuel, 2007, 86, 1021–1035 CrossRef CAS.
- A. Abad, T. Mattisson, A. Lyngfelt and M. Ryden, Fuel, 2006, 85, 1174–1185 CrossRef CAS.
- M. Johansson, T. Mattisson and A. Lyngfelt, Chem. Eng. Res. Des., 2006, 84, 807–818 CrossRef CAS.
- H. J. Ryu, G. T. Jin, D. H. Bae and C. K. Yi, Continuous Operation of a 50 kWth Chemical-Looping Combustor: Long-Term Operation with Ni- and Co- Based Oxygen Carrier Particles, http://lib.kier.re.kr/balpyo/clean5/13.pdf, Accessed 5 May, 2008 Search PubMed.
- P. Kolbitsch, J. Bolhàr-Nordenkampf, T. Pröll and H. Hofbauer, in The 9th International Conference on Circulating Fluidized Beds,Hamburg, Germany Editon edn, 2008 Search PubMed.
- M. Ishida and H. G. Jin, Energy, 1994, 19, 415–422 CrossRef CAS.
- J. F. Elliott, R. M. Smailer, R. L. Stephenson and Iron and Steel Society of AIME., Direct reduced iron: technology and economics of production and use, Iron & Steel Society of AIME, Warrendale, PA., 1980 Search PubMed.
- P. Gupta, L. G. Velazquez-Vargas, C. Valentine and L. S. Fan, Rev. Sci. Instrum., 2007, 78 Search PubMed 085106.
- L.-S. Fan, F. Li, L. G. Velazquez-Vargas and S. Ramkumar, Proceedings of the 9th International Conference on Circulating Fluidized Beds, Hamburg, Germany, 2008 Search PubMed.
- L. S. Fan, F. Li and S. Ramkumar, Particuology, 2008, 6, 131–142 CrossRef.
- G. Tomlinson and D. Gray, Chemical Looping Process in a Coal-to-Liquids Configuration. DOE Report, USDOE/NETL-2008/1307, NETL, US Department of Energy, 2007 Search PubMed.
- H. J. Ryu and G. T. An, Korean J. Chem. Eng., 2007, 24, 527–531 Search PubMed.
- G. T. Jin, H. J. Ryu, S. H. Jo, S. Y. Lee, S. R. Son and S. D. Kim, Korean J. Chem. Eng., 2007, 24, 542–546 Search PubMed.
- K. Svoboda, A. Siewiorek, D. Baxter, J. Rogut and M. Pohorely, Energy Convers. Manage., 2008, 49, 221–231 CrossRef CAS.
- K. Svoboda, G. Slowinski, J. Rogut and D. Baxter, Energy Convers. Manage., 2007, 48, 3063–3073 CrossRef CAS.
- W. U. Xiang and Y. Y. Chen, Energy Fuels, 2007, 21, 2272–2277 CrossRef CAS.
- WO Pat., 2 007 002 792, 2007 Search PubMed.
- M. V. Iyer,PhD Thesis, The Ohio State University, 2006 Search PubMed.
- K. B. Lee, M. G. Beaver, H. S. Caram and S. Sircar, Adsorption, 2007, 13, 385–397 CrossRef CAS.
- K. B. Lee, M. G. Beaver, H. S. Caram and S. Sircar, J. Power Sources, 2008, 176, 312–319 CrossRef CAS.
- H. Zhao, L. Liu, B. Wang, D. Xu, L. Jiang and C. Zheng, Energy Fuels, 2008, 22, 898–905 CrossRef CAS.
- J. Yang, N. Cai and Z. Li, Energy Fuels, 2007, 21, 3360–3368 CrossRef CAS.
- S. A. Scott, J. S. Dennis, A. N. Hayhurst and T. Brown, AIChE J., 2006, 52, 3325–3328 CrossRef CAS.
- Y. Cao, B. Casenas and W. P. Pan, Energy Fuels, 2006, 20, 1845–1854 CrossRef CAS.
- Y. Cao and W. P. Pan, Energy Fuels, 2006, 20, 1836–1844 CrossRef CAS.
- B. M. Corbella and J. M. Palacios, Fuel, 2007, 86, 113–122 CrossRef CAS.
- P. Gupta, L. G. Velazquez-Vargas, T. Thomas and L.-S. Fan, Proceedings of 29th Clear Water Coal Conference, Clear Water, Florida, USA, 2004 Search PubMed.
- P. Gupta, L. G. Velazquez-Vargas, F. Li and L.-S. Fan, Proceedings of 23nd Annual International Pittsburgh Coal Conference, Pittsburgh, Pennsylvania, USA 2006 Search PubMed.
- S. Y. Lin, M. Harada, Y. Suzuki and H. Hatano, Energy Convers. Manage., 2005, 46, 869–880 CrossRef CAS.
- K. Kuramoto, S. Shibano, S. Fujimoto, T. Kimura, Y. Suzuki, H. Hatano, S. Y. Lin, M. Harada, K. Morishita and T. Takarada, Ind. Eng. Chem. Res., 2003, 42, 3566–3570 CrossRef CAS.
- S. Y. Lin, M. Harada, Y. Suzuki and H. Hatano, Fuel, 2006, 85, 1143–1150 CrossRef CAS.
- S. Y. Lin, M. Harada, Y. Suzuki and H. Hatano, Fuel, 2004, 83, 869–874 CrossRef CAS.
- R. G. Rizeq, R. K. Lyon, V. M. Zamansky and K. Das, Proceedings of the International Technical Conference on Coal Utilization & Fuel Systems, 2001 Search PubMed.
- R. G. Rizeq, J. West, A. Frydman, R. Subia, R. Kumar and V. Zamansky, Fuel-Flexible Gasification-Combustion Technology for Production of H2 and Sequestration-Ready CO2, DOE Report, 2002. http://www.osti.gov/bridge/servlets/purl/832843-L11t1m/native/832843.pdf, Accessed 5 May, 2008 Search PubMed.
- H. A. E. Andrus, Jr., J. H. Chiu, G. N. Liljedahl, P. T. Stromberg, P. R. Thibeault and S. C. Jain, Proceedings of the 22nd Annual International Pittsburgh Coal Conference, Pittsburgh, Pennsylvania, USA, 2005 Search PubMed.
Footnote |
| † Key Assumptions for Fig. 16: Illinois #6 coal is used in all cases; For SCL, Syngas–CLC, IGCC–Selexol, and Gasification–WGS, a GE quench gasifier is used. A GE 7H gas turbine combined cycle system is used to generate electricity; Sub-critical plant operates at 17.5 MPa/538 °C/538 °C, ultra-supercritical plant operates at 26 MPa/600 °C/600 °C; CO2 is compressed to 15.20 MPa (150 atm) for sequestration. |
| This journal is © The Royal Society of Chemistry 2008 |