Electrospun nanofibers in energy and environmental applications
V.
Thavasi†
*a,
G.
Singh†
a and
S.
Ramakrishna
*abc
aNanoscience and Nanotechnology Initiative, National University of Singapore, 2, Engineering Drive 3, Singapore, 117576, Singapore. E-mail: seeram@nus.edu.sg; nnitv@nus.edu.sg
bDepartment of Mechanical Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore, 117576, Singapore
cDivision of Bioengineering, National University of Singapore, 9 Engineering Drive 1, Singapore, 117576, Singapore
First published on 23rd July 2008
Abstract
Nanotechnology is providing new solutions and opportunities to ensure sustainable energy and environments for the future. Materials of nanofiberous morphology are attractive to solve numerous energy and environmental issues. Nanofibers can be effectively produced by electrospinning, which is a simple and low cost technique. In addition, electrospinning allows the production of nanofibers from various materials e.g. organics and inorganics in different configurations and assemblies. This is highly beneficial for energy devices, where inorganic materials especially metal oxides can be synthesized and electrospun, improving conducting and ceramic properties. Excitonic solar cells fabricated with aligned nanofiberous metal oxide electrodes provide higher solar–electric energy conversion efficiency, whereas fuel cells made with nanofiberous electrodes enable uniform dispersion of catalysts, and thus increase electrocatalytic activity to obtain higher chemical–electric energy conversion efficiency. The nanofibers used in filtration membranes for environmental remediation, minimize the pressure drop and provide better efficiency than conventional fiber mats. The large surface area-to-volume ratio of nanofiber membranes allows greater surface adsorption of contaminants from air and water, and increases the life-time of the filtration media. This review highlights the potential and application of electrospun nanofiberous materials for solving critical energy and environmental issues.

| Dr Velmurugan Thavasi (left) is a Research Fellow at the NUS Nanoscience and Nanotechnology Initiative. He obtained a MEng in Chemical Engineering and a PhD in Chemistry from National University of Singapore. His research interests include synthesis of 1-dimensional nanostructured materials and design of excitonic solar cells and bio-solar cells. |
Dr Gurdev Singh (right) is a Research Fellow at the NUS Nanoscience and Nanotechnology Initiative. He obtained a PhD in Civil Engineering from the National University of Singapore. His research interests include membrane processes, environmental remediation and the application of nanoscience in environmental technology. |
Prof. Seeram Ramakrishna (middle) is Vice President (Research & Strategy) of the National University of Singapore. He holds positions on many advisory boards, including companies, universities and journals. He received his PhD from the University of Cambridge. He is among the most cited researchers (top 3%) working in the field of materials and electrospinning, and nanofibers engineering. He was among the first scientists to realize the potential applications of nanofibers in the fields of energy, environment, healthcare, and defense security. |
1. Introduction
Energy and environment head the list of top global problems facing society for the next 50 years. At present, fossil fuels are the primary source used to meet the energy demands of humanity, however, these resources are finite. Furthermore, processing of fossil fuels leads to global warming due to emissions of greenhouse gases, including carbon dioxide, methane, nitrous oxide, and other gases, such as volatile organic compounds, and hydrofluorocarbons. The quality of the environment has also deteriorated due to industrialization, which releases many pollutants into the atmosphere.1Water and air represent two environmental systems where the most pressing environmental issues persist. Water pollution and dwindling freshwater supplies are often cited as critical global problems. It is estimated that more than 50% of nations in the world will face freshwater stress or shortages by 2025. By 2075, it is further estimated that the number of nations facing these problems will increase to become 75% of all nations.2 Similarly, air is the other environmental system that is of concern. Generally, air can be characterized as indoor air i.e. within buildings and indoor environments e.g. vehicles, and outdoor air. Interestingly, when compared, indoor air quality is a more acute concern as it tends to have higher concentrations and prevalence of contaminants than outdoor air.3 As a result, more health problems generally arise from exposure to indoor air than outdoor air.Given the recognized threats to the world's collective energy security and environment, the focus must be redirected, as quickly as possible, towards addressing these critical challenges and driving global research to develop technology and devices for clean energy—conversion, storage and conservation; and a clean environment—water and air standpoints.
Researchers have been investigating clean energy opportunities such as, using solar energy more efficiently and cost effectively for generation of electricity, electrolysis of water to generate hydrogen and into electricity with reduced emissions viafuel cells. Efficient use of energy is often connected with energy conservation. Once the electricity is generated it must be efficiently stored during low demand periods, or for use in portable applications. This highlights the need for electrochemical energy storage devices, via high energy density batteries and/or super capacitors. Then, the storage of hydrogen becomes of interest, particularly when considering the use of hydrogen as fuel for electric vehicles or as a clean burning fuel for vehicles with combustion engines. While in the broadest sense, processes involved in energy and environmental treatment technology, can be considered in one way or the other, charge generation, transport and collection, as well as diffusion. Common challenges are improved performance, conversion efficiency, energy/power density, discharge rate and extended life with reversibility, and reduced production and operation costs. In particular, rapid e−, Li+, and H+ transport are essential for achieving desired performance in high efficiency solar cells, high energy density batteries and high efficiency fuel cells, respectively. A potential solution to overcome the shortage of water is to tap alternative sources of water, such as seawater, rainwater, wastewater effluent etc. However, contaminants in these waters have to be removed and water quality improved, before the water can be fit for usage. Indoor and outdoor air have to be purified; it was estimated that the global market for filtration and separation products reached US$37.3 billon in 2007/8.4 Thus, both water and air quality systems have to be controlled to ensure a safer environment by implementing efficient technology.
The demand for such high performance devices or efficient technology has led to increasing attention in advanced functional nanosized materials. It is only recently that attention has been given to the 1-dimensional (1D) morphology that is used for high technology applications. 1D semiconducting nanowires, nanofibers and nanotubes and also patterning of materials can be used to enhance charge transport and as building blocks in energy and electronic devices because of their confinement effects. Water and air purification or removal of contaminants can be effectively achieved using nanofiber membranes. Though nanofiberous materials can be prepared by methods such as: template-directed,5 vapor-phase approach6 solution-liquid-solid (SLS) technique,7solvothermal synthesis,8 solution-phase growth based on capping reagents,9 and self-assembly,10electrospinning (ES) has become more popular because of its easiness and low cost. Electrospinning (ES) is a physical process and moreover, one of the available inexpensive industry viable technologies, which can truly produce 1D fibers of μm to nm size ranges in diameter.11 The broad applications of electrospinning technology are summarized in Table 1. A quick analysis of nanofibers use for advanced functional applications over the past 10 years (Fig. 1) has indicated that their impact has been substantial. In particular, the impact of nanofibers in energy conversion (solar cells and fuel cells), and also in water and air treatment applications have been realized well and is encouraging (Fig. 2). This review highlights the potential and application of electrospun fiberous materials for energy and environmental treatment applications.

|
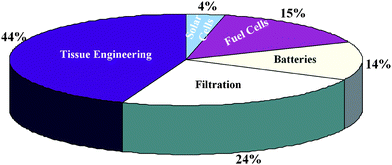 | ||
| Fig. 1 Statistics on the literature published on the advanced applications of nanofibers. (Search made through Medlink database.) | ||
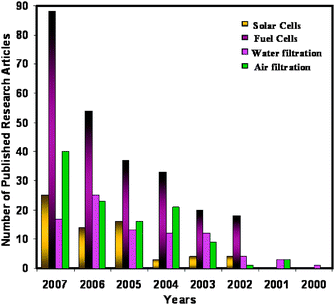 | ||
| Fig. 2 Research articles published on nanofibers' applications in energy devices and environmental treatment. | ||
2. Principle of electrospinning
Electrospinning of materials involves the application of a strong electric field to a droplet of a fluid, such as a melt or blend solution. A schematic of an electrospinning set up is shown in Fig. 3. The set up mainly consists of a spinneret, collector and high voltage power supply. A potential (in kV) is applied between the spinneret and a collector; both are electrically conducting and separated at an optimum distance between the two. The interactions of the electrical charges in the polymer fluid with the external electric field causes the pendant droplet to deform into a conical structure called the Taylor cone (Fig. 3(a)) and a critical voltage is attained. When the applied voltage surpasses the critical value at which repulsive electrostatic forces overcome the surface tension, a fine charged jet is ejected from the tip of the Taylor cone. These charged jets undergo a whipping motion and elongate continuously via electrostatic repulsion until they are deposited onto a grounded collector; resulting in the formation of fine fibers. Instability can occur if the applied external electrostatic field is not above the critical value, which would cause the jet to break up into droplets. Such phenomena is called Rayleigh instability. Therefore, the formation of nanofibers is a function of operating parameters viz. applied voltage, solution feeding rate and solution properties, mainly conductivity, viscosity and surface tension. Consequently, these electrospinning process parameters can be tuned to produce a wide range of fiber diameters.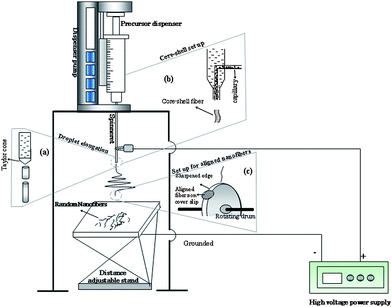 | ||
| Fig. 3 Schematic of electrospinning set up to obtain random nanofibers. (a) Enlarged view of formation of fiber starting from the Taylor cone. (b) Set up to obtain aligned nanofibers using rotating drum with sharp edge. (c) Set up to obtain core-shell nanostructures. | ||
In general, two types of 1D nanostructures can be formed using electrospinning: aligned and non-aligned nanofibers (Fig. 4). Electrospinning by simple methodology produces nonwoven form nanofiber layers or mats, which can be useful for applications such as filtration, tissue scaffolds, and implant coating films. Electrospinning with improved design or additional set up, such as a rotating disc or cylinder, could produce aligned nanofibers (Fig. 3(c)) or uniaxial fiber bundles that can be more useful in energy conversion devices. Geometry and rotating speed of a disc, or optimization of the electric field enable one to obtain the aligned electrospun nanofibers. The rotation of a cylinder collector at a very high speed, up to thousands of revolutions per minute, could result in electrospun nanofibers being oriented circumferentially.12–14 Researchers have also introduced insulators to influence the electrostatic forces acting on a charged fiber and force them to align the fibers uniaxially.15,16 Controlling the distance between the electrodes should also form aligned fibers, however, which is to be determined by the molecular weight of the polymer. For instance, Sundaray et al.17 obtained aligned polymethylmethacrylate (PMMA, Mw = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) polymer fibers, with separation between the fibers in the range of 5–100 mm by controlling the distance between the electrodes at about 1–3 cm, whereas 15 cm is the distance between the electrodes needed to obtain an aligned high molecular weight of polystyrene (PS) fibers. Core-shell nanofibers can be fabricated by co-electrospinning two different polymer solutions through a spinneret comprising two coaxial capillaries as shown in Fig. 3(b).18,19 Uniform nanofibers of conjugated polymers have also been prepared via a coaxial electrospinning technique.20,21
000) polymer fibers, with separation between the fibers in the range of 5–100 mm by controlling the distance between the electrodes at about 1–3 cm, whereas 15 cm is the distance between the electrodes needed to obtain an aligned high molecular weight of polystyrene (PS) fibers. Core-shell nanofibers can be fabricated by co-electrospinning two different polymer solutions through a spinneret comprising two coaxial capillaries as shown in Fig. 3(b).18,19 Uniform nanofibers of conjugated polymers have also been prepared via a coaxial electrospinning technique.20,21
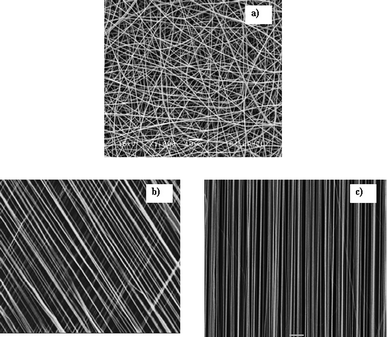 | ||
| Fig. 4 Scanning electron micrographs of electrospun (a) random nanofibers, (b) aligned fibers at an angle, (c) aligned fibers. | ||
3. Energy conversion devices
3.1 Dye-sensitized solar cells
Electrical energy is the most versatile energy for the 21st century world. Technologies are under development to obtain electrical energy from renewable sources, such as sun, wind, tidal etc. Photovoltaics is one of these technologies, it converts solar light directly into electrical energy, has reached several stages of development and is promising because solar energy is, almost, considered as an inexhaustible supply of energy. For the first time Regan et al.22 showcased the potential of nanotechnology to obtain electricity by constructing a dye-sensitized solar cell (DSSC). DSSC technology is an ‘artificial photosynthesis’ process using an electrolyte, a film of metal oxide nanoparticulate as the electrode and a dye sensitizer sandwiched between conducting glasses. In a DSSC, a light harvesting material (dye) generates excitons (bound electron-hole pairs) upon absorption of photons, and undergoes dissociation to release them as free electrons and holes. The free electrons are injected into the metal oxide nanoparticulate and transported for collection at the electrode. The more the electrons collected, the higher the efficiency. Researchers have used spin coating,23–25 screen-printing,26 doctorblading,27 and chemical vapour deposition28 to form titania nanofilms as a photoelectrode. Researchers recently have realized that grain boundaries diminish the efficiency of electron conduction in nanoparticulate matrix intemperately, and lead to charge–carrier recombination. The 1D morphology of the metal oxide fibers is believed to contribute to better charge conduction, because of their reduced grain boundaries compared to those of sintered nanoparticles, and also provides high specific surface area for the increased adsorption of dye sensitizers.29Polymers such as polystyrene (PS), polymethylmethacrylate (PMMA), polyvinylpyrrolidone (PVP) and polyvinyl acetate (PVAc) have been used to prepare such metal oxide nanofibers. Compared with other polymers, PVP is readily soluble in safe solvents, such as water, and produces a prolific amount of electrospun material, suggesting that it has the potential for large-scale ceramic fiber production.30 While replacing the liquid electrolytes with solid or semi-solid type electrolyte in order to attempt for flexible solar cells, imperfect filling of the electrolyte in the cells has been an issue. Nanofiberous metal oxide fabricated by the electrospinning method has larger pores than metal oxide as nanoparticles,29 this is expected to enhance the penetration of viscous polymer gel electrolyte. The solar–electric energy conversion efficiency which has been obtained from electrospun TiO2electrodes with a poly(vinylidenefluoride–co–hexafluoropropylene) (PVDF–HFP) gel electrolyte is over 90% of that from a liquid electrolyte system,31,32 which confirms the effective penetration of viscous gel electrolyte due to the porous morphology. The major problem of using nanofiberous inorganic electrodes in DSSCs is their poor adhesion to substrates after calcination, which is carried out to remove binder polymers. Due to the high temperature used for the calcination process, strong stresses are generated, and lead to the shrinkage of the fibrous mats. Such shrinkage has limited the application of electrospun ceramic nanofibers film, especially for metal oxide electronic devices TiO2 webs which were found to peel off substrates. Song et al.33 have used hot press pre-treatment to improve the adhesion of the TiO2 nanofibers onto the substrate. Onozuka et al.29 introduced solvent vapour to relax the nanofiber mats that, also, resulted in improved adhesion between TiO2 nanofibers and the conducting substrate. Fujihara et al.34 transformed electrospun nanofibers into nanorods by mechanical grinding in order to eliminate the adhesion difficulties of longer nanofibers developed by the electrospinning technique with the conductive glass plate (Fig. 5 and 6). DSSCs fabricated using such nanorods under air mass 1.5 global filter (AM1.5 G) condition delivered a current density 13.6 mA cm−2, open circuit voltage 0.8 V, fill factor 51% and energy conversion efficiency 5.8%. Recently, our group introduced an ultra-thin surface treatment layer (STL) on the conducting substrate before the deposition of the electrospun TiO2 nanofibers, which retained adhesion of nanofibers on the conductive substrate even after calcination.160 After calcination, the STL acted as an adhesive and thus bettered the adhesion of TiO2 nanofibers.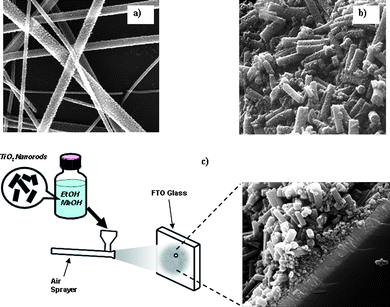 | ||
| Fig. 5 Scanning electron micrographs of (a) TiO2 nanofibers prepared in our laboratory. (b) TiO2 rod electrode made by mechanical grinding of brittle TiO2 nanofibers. (c) Spraying of TiO2nanorods onto the surface of FTO glass to make a photoanode for a DSSC. | ||
 | ||
| Fig. 6 (a) Scanning electron micrographs of ZrO2nanorods prepared in our laboratory after grinding the electrospun nanofibers. (b) Magnified micrograph of ZrO2nanorod with average diameter of 433 nm. | ||
Doping of materials has also been successful in advancing the function of materials.35–38 For example, strontium doped TiO2 (SrTiO3) has reduced the charge–carrier recombination in DSSCs.39 Doping the TiO2 with erbia showed emitting property in the near-infrared emission spectra range 6000–7000 cm−1 and, therefore, is used as a selective emitter for thermophotovoltaic applications. Tomer et al.40 prepared the erbia-containing titania electrospun nanofibers using erbium(III) oxide particles, tetraisopropyl titanate and 10% solution of PVP as precursors at 1 kV. They obtained mechanically stable nanofibers and also noticed that the large surface to volume ratio characteristics of nanofibers allowed gases to pass through with a lower pressure drop than materials made from micron-sized fibers.
3.2 Organic solar cells
Organic photovoltaic (PV) devices based on blends of conjugated polymers and inorganic nanostructures are currently objects of intense research for low cost solar energy conversion. Organic cells utilize a bulk heterojunction of donor and acceptor materials to provide a large internal surface area for the efficient charge separation of photo-generated excitons. However, such devices are limited by inefficient charge transport because of the highly folded, discontinuous topology of the donor–acceptor (DA) interface. Annealing could improve the transport process, however, may not be compatible with the use of most of the polymers, which have a low glass transition temperature. Therefore, alternative processing procedures that yield the same nanomorphology without thermal treatment are necessary for the development of efficient and flexible solar cells. Replacing the disordered phase with an aligned array of nanowires could improve charge transport and increase power conversion efficiencies without any thermal post treatment.41 However, most of the conjugated polymers are difficult to electrospin because of their lower molecular weight and solvent limitation. For instance, polypyrrole (PPy), a conducting polymer, has attracted more attention because of its unique electrical, optical, and photoelectric properties as molecular wires and molecular devices42 but exerts poor solubility in common solvents, due to its strong inter- and intra-chain interactions. Recently, methods have been developed to produce the fibers from such lower or solvent sensitive polymers using electrospinning. Chronakis et al.43 have improved the processability of PPy by mixing with poly(ethylene oxide) (PEO) and electrospinning to form ultrafine fibers. By increasing the PPy content of the PPy/PEO nanofibers, a higher electrical conductivity has been achieved. Also, the higher concentration of PEO in the initial solution could allow for easy stretching of fibers; that provides higher conductive pathways or charge–carrier mobility of PPy molecules along the fibers.44 The resulting nanofibers showed better electrical conductivity of approximately 3.5 × 10−4 S cm−1 for 50 wt% PPy precursor [PPy(SO3H)–DEHS] and 1.1 × 10−4 S cm−1 for 37.5 wt% of PPy precursor.3. 3 Hybrid solar cell
Hybrid materials such as inorganic–polymer composites, or encapsulated functional materials have shown promise in achieving the higher performance of energy and electronic devices.45Polymer–metal oxide nanoscale composites play a role in photovoltaic cells.46 Recently, 1D nanostructures of organic–inorganic hybrid composites have been successfully made viaelectrospinning.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM was added to the ZnO nanofibers, a dramatic increase in the external quantum efficiency (EQE) was observed to a value over 57% at 515 nm. This increase is likely to be due to enhanced excitons' dissociation in the film. It has been demonstrated that the interface of nanofiberous ZnO/P3HT
PCBM was added to the ZnO nanofibers, a dramatic increase in the external quantum efficiency (EQE) was observed to a value over 57% at 515 nm. This increase is likely to be due to enhanced excitons' dissociation in the film. It has been demonstrated that the interface of nanofiberous ZnO/P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM produces the conversion efficiency (η) of 2.03%.48 Solar cells fabricated with vertically aligned nanowire morphology are expected to deliver very high performance by suppressing electron–hole recombination and effectively improving the transport, however, it has not yet done so and is even inferior than random fibers.49–51 This may be due to uncontrolled spacing of vertically aligned nanofibers obtained by the solution grown method, which is usually in the range of 100 nm. It is to be noted that the typical exciton diffusion length of polymer P3HT is about 10 nm or even lower. Hence, closer nanofibers spacing must be necessary to increase the excitons' dissociation and achieve larger short circuit current (Isc). Electrospinning has not been engineered, so far, to produce the vertically aligned nanofibers. Nonetheless, such a breakthrough can be expected in the near future to solve such a critical issue.
PCBM produces the conversion efficiency (η) of 2.03%.48 Solar cells fabricated with vertically aligned nanowire morphology are expected to deliver very high performance by suppressing electron–hole recombination and effectively improving the transport, however, it has not yet done so and is even inferior than random fibers.49–51 This may be due to uncontrolled spacing of vertically aligned nanofibers obtained by the solution grown method, which is usually in the range of 100 nm. It is to be noted that the typical exciton diffusion length of polymer P3HT is about 10 nm or even lower. Hence, closer nanofibers spacing must be necessary to increase the excitons' dissociation and achieve larger short circuit current (Isc). Electrospinning has not been engineered, so far, to produce the vertically aligned nanofibers. Nonetheless, such a breakthrough can be expected in the near future to solve such a critical issue.
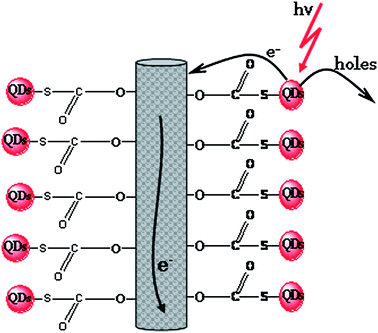 | ||
| Fig. 7 Structurally ordered and stable covalent (linker) attachment of quantum dots onto the nanofibers. | ||
3. 4 Fuel cells
Fuel cells are devices where electrochemical oxidation of hydrogen or hydrogen-rich fuel is converted in the presence of a metal catalyst into electrical current. As they produce clean energy in the form of current, they do not need recharging or replacing, so the research for higher efficiency and high capacity fuel cells has been intensified in recent years.57–61 A fuel cell consists of an electrolyte sandwiched between anode and cathode materials. The type of electrolyte distinguishes that fuel cell from other types of fuel cells. Polymer electrolyte membrane (PEM) and methanol fuel cells are being developed for automobile propulsion and as portable electronic devices, such as personal digital assistants (PDAs), cell phones, and notebook computers. The direct methanol fuel cell (DMFC) is very attractive since it operates at room temperature, uses liquid methanol as the fuel, and has the potential to deliver high energy density.62 In DMFCs, aqueous methanol is electrooxidized by metal catalysts, Pt63 or PtRu,64,65 to produce electrical current. The schematic of PEM is shown in Fig. 8.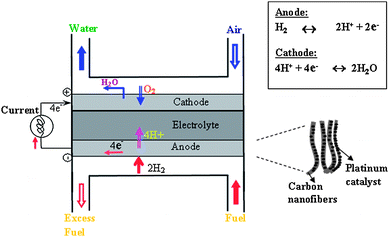 | ||
| Fig. 8 (a) Schematic of PEM fuel cell. The proton exchange membrane as electrolyte allows only the positively charged ions to pass through. The negatively charged electrons travel along an external circuit as current. (b) Schematic of nanofiberous carbon electrode structure embedded with catalysts. | ||
Fuel cells typically need higher platinum (Pt) content (20–60 wt% Pt) as the anode catalyst and in a thin layer to minimize the ohmic resistance and mass transfer limitations.66 Recent research in fuel cells has been aimed at reducing the amount of catalyst used, to even as low as 0.1 mg Pt cm−2 or below because of the expense of noble metal catalysts, but still maximizing anode catalyst utilization. To improve the anode catalyst performance, catalytic supporting materials must be stable and dispersed uniformly. To achieve the uniform dispersion, porous nanomaterials having a larger surface area are used as electrode materials. For stability, supporting materials have been considered for the catalyst. Polymers such as polyaniline nanofibers (PANI) have been considered as the most promising matrix for dispersing the Pt nanoparticles.67 The porous network structure of PANI has been effective for dispersion of Pt particles (about 10–20 nm) and facilitates easy access of precursor (fuel) to the catalytic sites.68 Moreover, PANI has a high accessible surface area, and is a good electron conductor.69Nanowires of conducting polymer are promising support matrices for the dispersion of Pt particles, as they provide conducting pathways and surface interactions between the nanoparticles and conducting polymers. Nanostructured PANI prepared by hard templates such as the alumina membrane method increases catalyst cost and makes scale-up difficult. Electrospinning techniques can be used to disperse the nanoparticles onto the nanofibers uniformly (Fig. 9) and moreover without the involvement of a surfactant or template. The large surface area in the nanofibrillar PANI has enabled the dispersion of catalyst particles with less time for the deposition of Pt particles.68 The electrocatalytic performance of methanol oxidation for PANI nanowires supported Pt composite has been found to be much higher than at bulk Pt electrodes.70
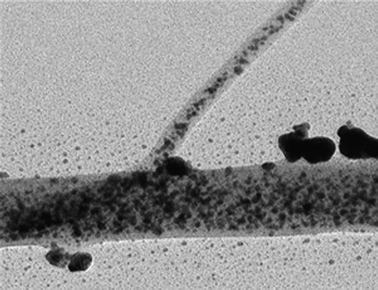 | ||
| Fig. 9 Scanning electron micrograph of electrospun PANI nanofibers with silver precursor prepared in our laboratory resulted in uniform dispersion with Ag nanoparticles. | ||
Proton exchange membrane fuel cells69,71,72 and direct methanol fuel cells73,74 have been found to deliver better performance when CNTs or CNFs were used as the support for the Pt catalyst in the fuel cell systems65,75,76 because of their high electrical conductive nature.77 Different approaches have been reported for introducing a porous structure into the bulk of an electrospun nanofiber such as selective dissolution,78 selective thermal degradation79 and liquid nitrogen freezing processes.80 Also, porous sizes can be successfully obtained by controlling process parameters such as the potential applied or drawing rate during the electrospinning process.81–83 Yuan et al.84 have suggested that the fuel cell performance using twisted CNFs as the anode was better than that of straight CNFs due to its specific surface conformation, which helps to disperse the Pt more homogeneously. The twist or alignment of CNTs in a polymer matrix can be achieved by applying and varying the electric potential which is possible during electrospinning. Nanofibers containing both SWCNTs and MWCNTs can be electrospun from a polymer-based solution. Sundaray et al.17 have obtained controlled preparation of long and aligned CNT nanofibers. Yasuda et al.85 have noted that increases in the carbon nanofiber content resulted in a random orientation, and increased Young's modulus but decreased hardness. It indicates that the mechanical properties can be influenced by the content of carbon nanofibers for electrospinning and therefore it should be the focus of more attention.
Researchers have also paid attention to developing green catalystsviz. enzymes or biomolecules instead of synthetic catalysts in order to reduce the cost or increase the performance. The geometrical effect i.e. the number of molecules per unit area (or volume) plays a predominant role when immobilizing biomolecules onto the materials as the actual size of such molecules is quite large. As such, the number per unit area (or volume) will not be so high for the nanoparticle layer, and therefore attempts to cross link or immobilize the enzyme onto the nanoparticulate layers may limit or even lose bio(enzyme) activity. Therefore, 1D structure of materials can be emphasized, not only to obtain more enzyme or biomolecule binding but also for controlled electron transport. Electrospun nanofibers have been used to bind or immobilize the catalytically active enzymes.86 Recently, Patel et al.86 have demonstrated horseradish peroxidase (HRP) enzyme immobilized mesoporous silica fibers. The silica nanofibers were prepared from tetramethyl orthosilicate–glucose–enzyme solution by electrospinning with an electric field of 20 kV and at a distance of 15 cm. D-glucose has also been used, not only to function as a non-surfactant template for controlling mesoporosity, but also to help spinning of the solution by increasing viscosity. In general, the activity of free enzymes decreases rapidly with increases in temperature because of thermal denaturation whereas Patel et al.86 noticed that the immobilized enzymes in silica nanofibers showed an increase in activity even up to 60 °C. This improved thermal stability has been suggested to be due to the space confinement in the host fiber matrix, which prevents protein unfolding. Furthermore, the activity of enzyme immobilized silica nanofibers with mesoporosity has shown 4-fold greater activity than conventional templated silica samples and 3-fold greater activity than HRP immobilized silica powders.86 Such dramatically improved features and the versatility of this electrospinning technique are a boon to biocatalytic applications in biofuel cells.
4. Energy storage devices
4. 1 Hydrogen storage
Concerns about global climate change and the need for sustainable development have renewed interest in hydrogen as a fuel because using hydrogen zero-carbon emission vehicles can be produced. A future that depends on hydrogen is inevitable and hence, efficient methodologies for producing hydrogen and storing hydrogen have been under investigation. Hydrogen can be stored in materials effectively in the form of metal hydrides. The hydrogen is adsorbed into the spaces or interstices between the atoms in the metal. Hydrogen adsorption strongly depends on the materials' porosity. The smallest pores provide the highest storage capacity and therefore hydrogen storage is dominated by small pores. Small pores (1 nm or below) are the most efficient for hydrogen sorption. Activated carbon, exfoliated graphite and fullerenes have promise for high storage capacity and, more over, they are preferred due to their low cost and easy availability in large amounts. The hydrogen uptake is proportional to surface area and pore volume. As such, nanostructured carbon materials such as carbon nanotubes and carbon nanofibers have shown high hydrogen storage capacity.87 Nanofiberous materials can be prepared for the desired pore size ranges using electrospinning by optimizing process parameters to make materials with attractive morphology for hydrogen storage. Furthermore, electrospinning could develop high performance hydrogen storage materials as it can produce well structured, highly ordered, and high aspect ratio nanoscale dimensions.4. 2 Supercapacitors
Lithium-transition-metal-oxides include LiCoO2, LiNiO2, LiMn2O4, LiMnO2, and LiV3O8, all are high performance cathode materials for lithium-ion batteries.88,89 LiCoO2, in particular, is one of the most promising cathode materials for commercial applications, such as lithium-ion rechargeable batteries due to its good capacity, high specific energy, good power rates, low self-discharge, and excellent life cycle.90,91 Even if lithium-ion batteries, which combine high power and energy density, appear as the most promising system, there is still a need to improve the performance of electrode materials to achieve the highest capacity. High surface area conducting nanofibers make electrospun materials attractive as electrodes for batteries or in electrochemical supercapacitor applications. Gu et al.92 have demonstrated that LiCoO2 nanofibers could exhibit a high initial charge and discharge capacity of up to 216 and 182 mA h g−1, respectively. The traditional method for preparing LiCoO2 can result in inhomogeneity and irregular grain growth, whereas, the sol–gel method provides pure material with good crystallizability, homogeneity and uniform size. Electrospinning techniques have been very successful for making inorganic nanofibers utilizing the sol–gel method. Lithium-ions (Li+) are transported between electrodes through the electrolytic medium during charge and discharge. Compared to conventional film based morphology, nanotubes and nanofibers are expected to have improved electrode properties due to their high surface areas and 1D nanostructure properties, and therefore should provide higher rates of electron transfer. In addition, their entangled network allows easy access of the ions to the electrode/electrolyte interface. Lithium chloride (LiCl·1H2O), cobalt acetate (Co(CH3COO)2·4H2O) and 10 wt% PVA have been used as precursors in the preparation of nanofibers of LiCoO2. A voltage of 12 kV was used to provide a dense web of LiCoO2 fibers, dried for 5 h at 701 °C under vacuum, followed by calcination at 400–700 °C. The electrospun nanofibers of LiCoO2 demonstrated improved electrode properties as the result of high surface area.93 This method may also be used to synthesize other cathode materials for lithium-ion batteries and, therefore, has good prospects for commercialization.93The capacitance of the electrical double-layer capacitor is a function of the specific surface area, pore volume, and resistivity of the sample. Researchers, in order to enhance the specific energy and power of supercapacitors, have been trying to develop materials, such as conducting polymers by modifying their structures and controlling pore size distribution. Polybenzimidazol (PBI), a candidate polymer exhibits excellent thermal/mechanical stability, is durable to chemicals, and gives a high carbon yield. Also, PBI-based electrospun fiber does not require stabilization because of its thermosetting property that originates from the high rigidity of the polymer chains. Therefore, researchers expect that PBI should provide a high carbon yield and cost savings. Recently, carbon nanofiber webs have been prepared94,95 through the electrospinning technique and offered the prospect of accelerating the application of these materials in electrical energy-storage systems. Researchers, therefore, have been investigating methodologies to develop PBI-based activated carbon nanofiber webs as a novel electrode material for electric double layer supercapacitors. Kim et al.96 have prepared homogeneously distributed PBI-based nanofibers with a diameter of about 250 nm from a solution of 20 wt% PBI in dimethyl acetamide using electrospinning. The resulting electrospun nanofiber web electrodes have a large BET specific surface area in the range of 500–1220 m2 g−1 which has exhibited higher capacitance.96
Pico et al.97 have investigated electrospun ruthenium oxide–carbon nanofiber composites as an electrode material for capacitor application. RuO2·xH2O–CNF composite fibers are simple composites, which require neither an inert binder nor an electric conductor to be added to show high electric conductivity, nevertheless, Ru is a rare-earth metal, which is toxic and expensive.97 Researchers recently have paid much attention to conducting polymers as electrode materials for supercapacitor applications as they are lower in cost compared with ruthenium oxide. Of these, polyaniline (PANI) has been most promising due to its low cost, high conductivity and stability. Sivakumar et al.98 have prepared electrospun PANI nanofibers and found that the higher surface area of nanofibers led to higher charge storage ability. In addition, the nanofibers morphology could permit rapid access of the electrolyte to the electrode which should result in high charge storage and fast charge–discharge processes.
5. Optoelectronics
Nanoscale gallium oxide (Ga2O3) with a large surface area to volume ratio provides special conduction and optical properties that find potential applications in optoelectronic devices including flat-panel displays and optical limiters for ultraviolet region.99,100 However, conventional methods to make nanostructures of these electronic materials usually need complex equipment, catalysts, and hence, prepared β-Ga2O3 using such a method could face contamination issues due to the presence of residues of the catalyst. Electrospinning, an alternative and cost-effective method for making nanofiberous metal oxides, should eliminate the possibility of contamination. Zhang et al.101 have prepared Ga2O3nanoribbons by electrospinning using a sol–gel containing Ga(NO3)3·8H2O and PVP. Polymers, such as oligo- and polyfluorene as well as their derivatives are also excellent candidates for optoelectronic applications because they exhibit excellent fluorescence quantum yields, high thermal–chemical stability, and significant charge carrier mobility.102 The prepared electrospun nanofibers of polyfluorene–nonconjugated polymer with a pore size of 50–150 nm has exhibited better luminescence efficiency. Strong stretching forces exerted during electrospinning is involved in the orientation of polymer chains along the long axis of fiber.20 Such oriented electrospun nanofibers of conjugated polymers have exhibited higher charge–carrier mobility15,103,104 and photoluminescence.105–107 Aggregation of polyfluorene in the electrospun fibers has also been found to be smaller than that of spin coated films due to the geometrical confinement of the electrospinning process and, consequently, resulted in higher luminescence efficiency.102 The electrospinning induces orientation along the axis of a fiber by strong stretching forces associated with electric potential.102Another metal oxide tantalum pentoxide (Ta2O5) has been widely investigated because of its promising application in MOM or MOS capacitors108,109 wave-guides.110 Dharmaraj et al.111 have recently demonstrated that tantalum pentoxide nanofibers can be conveniently prepared by electrospinning. The precursor, tantalum isopropoxide with PVAc (11.5 wt%) is electrospun into nanofibers by applying a high voltage of 16 kV and keeping 17 cm as the distance. Calcined nanofiber mats lead to the formation of porous and pure tantalum oxide nanofibers.111
6. Transistors
The electrospun nanofibers of binary blends of conjugated polymers have tunable, composition-dependent, optical, and electronic properties that can be exploited in field-effect transistors (FET). Tunable luminescence characteristics can be demonstrated on the electrospun nanofibers of conducting polymers. Recently, the morphology and photophysical properties of electrospun nanofibers based conducting polymers have been studied for field effect transistor devices. Babel et al.20 have investigated the electronic properties of electrospun nanofibers of MEH–PPV with regioregular P3HT (Mw = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400), which were prepared by electrospinning using a coaxial two-capillary spinneret arrangement. For MEH–PPV–P3HT blend fibers, the P3HT is dispersed in the nanofiberous matrix of MEH–PPV as particles. The study demonstrated that MEH–PPV–PHT blend nanofibers exhibit p-channel transistor characteristics with hole mobility in the range of (0.05–1) × 10−4 cm2(Vs)−1 as nonwoven mats.20 Efficient energy transfer and enhanced red emission from P3HT have been observed in MEH–PPV–PHT blend nanofibers which clearly indicate that stronger interaction occurs when compared to bulk thin films due to confined nanofiberous structures. Davies et al.30 have shown that yttria stabilized zirconia can be successfully electrospun from a sol–gel precursor (ZrOCl2·8H2O and PVP) and that annealing at 1500 °C for 1 h resulted in a nanofiberous structure of pure zirconia. The fiber diameter was found to decrease with increases in annealing temperatures, and when average grain size increased the conversion of tetragonal to monoclinic structure occurred.30 Similarly, the anatase to rutile phase transition temperature observed by Kumar et al.112 for TiO2 nanofibers was found to be dependent on the nanoparticles' size and packing density (Fig. 10). The lower the fiber diameter, the higher the surface area would be, which suggests that interaction between the surface charges and the forces that hold the particle are higher for fibers of smaller diameter. The absorption spectra of the fibers showed a red shift with an increase in fiber diameter. The excitonic peak showed a shift of 15 nm when the diameter of the fiber increased from 60 to 150 nm.112 Thus, the diameter should determine the electronic property in electrospun nanofiberous metal oxide ceramics.
400), which were prepared by electrospinning using a coaxial two-capillary spinneret arrangement. For MEH–PPV–P3HT blend fibers, the P3HT is dispersed in the nanofiberous matrix of MEH–PPV as particles. The study demonstrated that MEH–PPV–PHT blend nanofibers exhibit p-channel transistor characteristics with hole mobility in the range of (0.05–1) × 10−4 cm2(Vs)−1 as nonwoven mats.20 Efficient energy transfer and enhanced red emission from P3HT have been observed in MEH–PPV–PHT blend nanofibers which clearly indicate that stronger interaction occurs when compared to bulk thin films due to confined nanofiberous structures. Davies et al.30 have shown that yttria stabilized zirconia can be successfully electrospun from a sol–gel precursor (ZrOCl2·8H2O and PVP) and that annealing at 1500 °C for 1 h resulted in a nanofiberous structure of pure zirconia. The fiber diameter was found to decrease with increases in annealing temperatures, and when average grain size increased the conversion of tetragonal to monoclinic structure occurred.30 Similarly, the anatase to rutile phase transition temperature observed by Kumar et al.112 for TiO2 nanofibers was found to be dependent on the nanoparticles' size and packing density (Fig. 10). The lower the fiber diameter, the higher the surface area would be, which suggests that interaction between the surface charges and the forces that hold the particle are higher for fibers of smaller diameter. The absorption spectra of the fibers showed a red shift with an increase in fiber diameter. The excitonic peak showed a shift of 15 nm when the diameter of the fiber increased from 60 to 150 nm.112 Thus, the diameter should determine the electronic property in electrospun nanofiberous metal oxide ceramics.
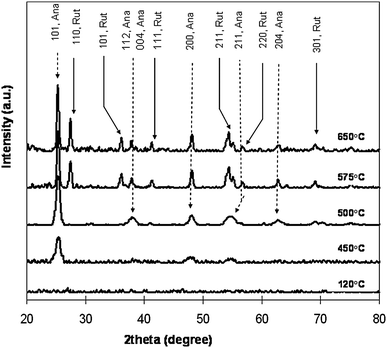 | ||
| Fig. 10 X-ray diffraction (XRD) patterns of electrospun TiO2 nanofibers showing the formation of anatase and rutile phases upon heat treatment. | ||
7. Environmental applications
It can be inferred that membrane or filtration systems offer a potential solution for a wide range of environmental issues. Therefore, it is no surprise that membrane technology and its industrial applications have expanded considerably in the last 50 years. Environmental purposes, such as water and wastewater treatment, as well as air purification exert the greatest demand on membrane usage. Slightly more than half of membrane usage is presently for water and wastewater applications.113Membrane processes are valued as a significant technological advancement for ensuring a sustainable future for mankind.114A membrane is defined as a semi-permeable barrier that allows only certain molecules and compounds access through it and denies passage to others. This is illustrated in Fig. 11 where only fluid is allowed to pass through the membrane and the contaminants in the fluid are prevented. The transport of these molecules or compounds across a membrane takes place by a sieving mechanism, or by diffusion of materials through it. Essentially, membranes are also referred to as filters that result in the separation of materials in a mixture. Rejected molecules or compounds can be prevented from passing through the membrane because of the tight pores at its surface. In this case the membrane functions as a screen filter. Alternatively, the molecules and compounds can also be rejected by being adsorbed or entrapped within the porous structure of the membrane in which case the membrane is referred to as a depth filter.
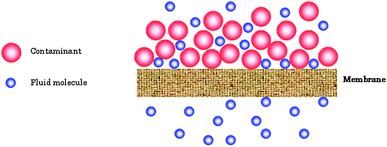 | ||
| Fig. 11 Schematic of filtration system where a nanofiber membrane is allowed to separate the containment from the fluid. | ||
Membrane processes are now well established technologies for remediation of environmental problems such as water and air treatment. However, membrane technologists are constantly striving to push the envelope of membrane performance i.e. to get more output with less input. To serve as an effective environmental technology or solution it is imperative that the process be as energy efficient as possible, since energy and environment are inexplicably linked. It is anticipated that the future of membrane technology lies in the development of more efficient and energy saving membranes. Nanofiber membranes made from electrospinning are potentially advanced membrane systems that can offer removal of pollutants from the environment at lower energy and hence cost. In the following sections we review some of the applications of nanofiber membranes for the environment, focusing on air purification and water treatment applications.
7. 1 Air filtration applications
Environmental applications of nanofiber membranes for air filtration started in the early eighties and has expanded in the last three decades. With industrialization, air quality has deteriorated in some places, requiring a regeneration of air through filtration and other processes. Furthermore, stricter emission limits have also contributed to the need for better quality filtration media. Air filtration spans a wide range of applications. From the removal of particulate materials from work environments, such as high dust loading industrial facilities to specialized applications, such as providing protection to individuals from toxic agents in the air.Today, nanofiber mats are used in numerous air filtration applications, either on their own or in combination with other filtration media. The growth in use of nanofibers for air filters have been pioneered by a few companies. These companies have been highlighted in previous reviews.115,116 Compared to conventional filtration media, nanofibers are of much smaller dimensions. The nanofibers used in filtration can be up to 800 times smaller than conventional filtration fibers. Smaller fibers generally provide better efficiency due to inertial impaction and interception, which makes up for the increased pressure drop when compared to bigger fibers.117 In addition, for nanometre sized fibers, the consideration of slip flow at the fiber surface implies a decrease in drag force on the fiber and therefore a reduced pressure drop. The occurrence of slip flow also results in more contaminants passing near the surface of the fibers, which increases the inertial impaction and interception efficiencies of the fibrous mat. Overall, this results in nanofiber mats that can achieve much higher efficiency for the same pressure drop as compared with conventional fiber mats. The cut-off diameter of the fiber for slip flow consideration at its surface is approximately 500 nm.118
This advantage and huge surface area to adsorb contaminants from air, have made nanofiber membranes an increasingly popular choice in air filtration applications. Below, we highlight some of the work that has been carried out or is being looked at in the application of nanofibers for air filtration.
7. 2 Dust capture applications
Dust refers to solid materials found in air with diameters smaller than 500 mm. Dust can come from natural sources such as soil swept up due to wind, from volcanic eruption or can be due to pollution. In most cases dust is a respiratory allergen but in some prolonged cases of exposure can even result in death, such as with the dust from coal mines. Dust capture is therefore an important process, to protect the individual particularly in enclosed spaces and is commonly incorporated in the ventilation system. Nanofibers based filters are increasingly being used in tandem with conventional filter media for dust capture purposes. Conventional filter media such as glass fibers exhibit depth loading characteristics when exposed to industrial dust environments. These media become clogged up over time and there is reduced airflow through the media as a result. When a nanofiber web is used, due to its smaller pore sizes, it is able to capture the dust particles on its surface. These particles can then be removed by back flushing or through other mechanical means. This implies that nanofiber based or nanofiber composite filters have a higher shelf life when compared with conventional filters. In comparison, other composite media do not exhibit as high permeability as the nanofiber filters.1197. 3 Vehicle cabin filters
Contamination by airborne contaminants or particulates in enclosed places where personnel work and operate in is an important concern. Examples of such places include cabins in machinery and equipment such as in mining work, airplane cabins, etc. Often wet-laid cellulose filter media is used to minimize the cabin dirt concentration, hence providing a safe environment for people in these areas.The effectiveness of nanofiber media in reducing the cabin contaminant concentration has been investigated using a composite cellulose/nanofiber filter and comparing it with a standard (wet-laid cellulose) filter.120 The filters have been tested independently on a Caterpillar 992G wheel loader, mounted with two particle sampling devices, one inside the cabin and the other outside. With the standard cellulose filter, a reduction in sub-micron and respirable (>1 μm) dust of 68% and 86%, respectively, has been noted. However, with the nanofiber composite filter a higher reduction of approximately 92% has been observed for both the sub-micron and respirable dust. Further tests revealed that the nanofiber filter life and initial pressure drop were comparable with the standard filters.121
7. 4 Building filters
Indoor air filtration is another important environmental concern. This is especially so, since we spend a large part of our lives in an indoor environment. From residential homes, to work offices, to hospitals, air filtration is an important requirement for ensuring a safe and refreshing environment to be in. Contaminants which can be circulated in these enclosed buildings can lead to severe health effects, the most common known as ‘sick building syndrome’. This is where occupants of a building, usually an office block, feel nauseous when inside the building. In hospitals where diseases are treated, the circulation of air can be tricky and filters which are able to prevent viruses and germs from spreading through the air circulation system are essential. If not prevented this could cause disease to spread throughout the hospital.Nanofiber membranes are a potential solution for indoor air filtration applications. Due to their high surface area and porosity, they not only capture the contaminants in the building circulation system but also allow more air to pass through. This implies that less energy is required to pump the air through the filters resulting in a sustainable solution. In addition, nanofibers can be functionalized to increase their capture efficiency or for specific applications, and to improve their properties e.g. make the filters antimicrobial. This will be discussed subsequently.
8. Personal respiratory protection from biological and chemical agents
Nanofibers are also increasingly being used for individual protection purposes. Their use in breathing masks has increased greatly in the last couple of years. Besides ensuring better breathability, they are also capable of removing very small particles such as viruses.122 With further functionalization of the nanofibers, improved performance can result from these materials. Our research group is engaged in the functionalization of the membranes for removal of certain persistent contaminants in the air. Some of these applications are highlighted below.Protection from biological and chemical agents is commonly achieved through protective suits and face masks. These are extremely important for the many personnel who are exposed to these agents on a frequent basis, such as personnel from the military, fire and rescue services and law enforcement. The major limitations of current protective materials are weight and moisture retention, which reduces the wearers' comfort during periods of prolonged usage.
The use of nanofiber webs in place of current materials e.g. activated carbon could reduce these problems. Firstly, nanofibers are light-weight materials, which can be easily embedded into current suits and face masks.123 Secondly, the nanofiber membranes can provide protection against aerosols and other liquid penetration. This is pertinent because chemical agents are commonly delivered as aerosols. In addition, through functionalization of the nanofiber membranes it is also possible to not only adsorb the biological and chemical agents but to decompose and detoxify the contaminants as well. This will potentially eliminate the tedious tasks of handling the hazardous used masks and suits.
Ramakrishna et al.124 have demonstrated that it is possible for functionalized nanofibers to be produced that can provide protection and detoxification of chemical warfare stimulants. To mimic enzyme functionalities, a chemical compound (3-carboxy-4-iodosobenzyl) oxy-β-cyclodextrin has been synthesized and electrospun with polyvinylchloride (PVC) into nanofibers. The functionalized nanofibers have been found to outperform activated carbon and even powerful nucleophiles e.g.iodobenzoic acid and β-cyclodextrin in detoxifying paraoxon an organophosphorous (acute neurotoxin) model.124,125 In another study, it has been found that ceramic zinc titanate nanofiber had a decomposition efficacy of between 77–91% for paraoxon (nerve agent) and 64–69% for CEES (2-chloroethyl ethyl sulfide) (mustard agent).
9. Liquid filtration applications
Nanofiberous media or membranes are increasingly being looked at as a solution for providing water at lower energy costs. Nanofibers due to their higher porosities and interconnected pore structures offer a higher permeability to water filtration over conventional materials being used. Below, some examples of nanofibers being used in liquid filtration are explored.9. 1 Removal of micron sized particles and suspended solids
Removal of micron sized and other suspended solid particles such as flocs, bacteria etc. is important in both water and wastewater treatment applications. Cryptosporidium parvum and Giardia lamblia are pathogenic protozoans whose symptoms include severe diarrohea, stomach cramps, nausea and vomiting. Following past outbreaks of diseases due to these protozoans and the lack of an effective treatment their removal from waters is now made compulsory in many countries.126Membrane processes such as microfiltration and ultrafiltration are well known to achieve high rejection rates of micron sized and other suspended solids from waters. These have become a popular means to achieve not only purification but disinfection of water to remove these pathogenic bacteria. Presently, MF and UF membranes are still being produced by the phase-inversion method127 and by dry formed methods for fibrous membrane media such as spun bonded and melt blown techniques.128 Electrospun membranes are another method through which these MF and UF membranes can be obtained and is postulated to grow significantly in the coming years.Kaur et al.129 have compared the filtration performance of a commercial MF membrane with an electrospun nanofiber membrane of the same polymeric material. The electrospun nanofiber membrane (ENM) had a water flux several times higher than the commercial membrane at the same applied pressure. However, the pore size of the ENM has been found higher when compared to the commercial membrane, therefore although it will produce a higher flux, it will also result in a smaller rejection for the same feed water through both membranes. To test if the nanofiber architecture could contribute to higher filtration flux at the same pore size, grafting has been carried out on the ENM to reduce its pore size. Graft-copolymerization with methacrylic acid has been carried out on the ENM, such that only the top layer of the nanofiber membrane has been modified and the nanofiber architecture maintained. The grafting reduced the average membrane pore size and bubble point to that of the commercial MF membrane. In addition, the contact angles of both the commercial and grafted ENM have also been found to be similar. The water flux of the grafted ENM has been found to be approximately 150–200% higher than the commercial membrane. This showed that the nanofiber architecture is better than the phase inverted and could result in energy saving membranes.
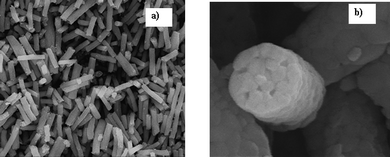
Gopal et al.130 have also explored the removal of micron sized particles using nanofiber membranes made from polysulfone. The membrane pore sizes could be adjusted by altering the electrospinning conditions. For comparison a nanofiber membrane of fixed pore size has been filtered for different polystyrene particles of various sizes under the same operating conditions. The rejection of the particles by the membranes decreased with the size of the particle. A clear change from the operation of the membrane as a screen filter to a depth filter has been noted as the particle size decreased during the experiments. More significantly, membrane fouling was the most severe when the particle sizes separated were close to the mean pore size of the membrane. This is likely to be due to the occurrence of pore blocking mechanisms. Fig. 12 shows a clean PSU nanofiber membrane and a fouled PSU nanofiber membrane after filtering particles of approximately 1 μm. The nanofiber membrane was able to effectively capture most of the particles passing through.
 | ||
| Fig. 12 SEM micrograph of (a) clean nanofiber membrane before filtration and (b) nanofiber membrane after filtration with 1 μm polystyrene particles. | ||
10. Heavy metal ion adsorption
Heavy metals are a serious biological problem in aquatic systems. Adsorption and filtration are the commonly used methods for removal of these contaminants. The advantage of nanofiber membranes is that they can offer both adsorption and filtration. Therefore, nanofiber membranes offer an attractive solution to the removal of heavy metals from water. In a recent study,131 silk fibroin and a blend of silk fibroin with wool keratose was electrospun into membranes and tested for the removal of heavy metal ions. It has been noted that the adsorption capacity was significantly enhanced when nanofibers (1.65–2.88 mg g−1) were used, as compared to conventional materials like wool silver (0.71 mg g−1) and filter paper (0.23 mg g−1) tested for removal efficiency of heavy metals. The higher adsorption has been attributed to the larger surface area of the nanofiber membrane.In another study to investigate the impact of impregnating boehmite nanoparticles into electrospun nanofibers, it has been found that a removal efficiency of approximately 0.20 mg g−1 for cadmium(II) in water could be achieved.132 It is anticipated that further optimization of the nanoparticles' concentration and the polymer could potentially achieve higher removal efficiency for heavy metal ions.
11. Adsorption of organic compounds
Organic materials in water can pose health hazards and need to be removed from drinking water. Functionalized nanofiber membranes offer a promising solution for the removal of organic molecules in water. Kaur et al.133 have studied the removal of phenolphthalein as a model organic molecule from water using a poly(methylmethacrylate) (PMMA) nanofiber membrane functionalized with phenylcarbomylated and azidophenylcarbomylated β-cyclodextrins. The membranes have been functionalized with the oligosaccharides by blending them in the PMMA solution and then electrospinning. The membranes were able to capture the phenolphthalein molecules, but could be further optimized to increase the adsorption capacity. Nevertheless, this exemplifies how nanofiber membranes can be used for the adsorption and removal of organic molecules in water systems.Humic acid is a commonly encountered natural organic matter in water. These compounds are considered to be polydisperse, structured polyelectrolytes of an amphiphilic nature.134 Our group attempted to utilize nanofiber membranes for the removal of humic substances from water. Nanofiber membranes on their own are able to adsorb some of the humic materials from water. However, the adsorption capacity and removal could be increased by functionalizing the membrane. In unpublished data, we blended surface modifying macromolecules with PVDF and electrospun them into nanofiber mats. We found that through this, humic acid rejection of 92% could be achieved at a pressure of only 0.25 psi. Further work is being carried out to functionalize the nanofiber membranes for removal of humic and other organic substances.
Another local group has explored using titanium dioxide nanofibers for the removal of humic acids.135 The advantage of TiO2 nanofibers is that they can function both as a filtration membrane and as a photocatalyst. Their results indicate that TiO2membranes when used as a photocatalyst can achieve almost 100% removal efficiency.
Oil is another organic material that is sometimes present in water sources due mostly to industrial discharge. The removal of oil has been investigated using a 3-tier membrane stack, with the middle tier composed of polyacrylonitrile (PAN) nanofibers.136 It has been found that the membrane system has been able to remove a high percentage of soyabean oil at a higher permeation rate compared with conventional ultrafiltration membranes.
Proteins are another group of commonly encountered organic compounds, particularly in wastewater applications. The removal of proteins has been achieved using polysulfone nanofiber membrane surfaces modified with carboxyl groups. The nanofiber membranes had an adsorption capacity of 17 μg mg−1 for bovine serum albumin (BSA), a model protein.137 Disinfection by-products (DBPs), which form due to the reaction between chlorine and organics, are potential carcinogens. Their removal is important in potable water. Our group have been focusing on the development of hydrophobic based nanofiber mats for the removal of these DBPs. Preliminary results have suggested that such high filtration flux nanofibers can achieve approximately 20–30% removal of DBPs. Further tests are being carried out to increase the rejection performance of the membranes.
12. Functionalized nanofibers with bactericidal effects
Nanofibers can also be functionalized to serve as potential antimicrobial filters in air and liquid filtration. In addition, the antimicrobial property of nanofibers is also desired when these are used in protective clothing and wound dressing applications. Silver ions and silver compounds have long been recognized as potent antimicrobial agents. Silver nanoparticles are increasingly being used in various antimicrobial applications, due to their increased surface area.138 The incorporation of silver nanoparticles into nanofibers offers synergies of two nanotechnologies. The silver nanoparticles can be incorporated onto the nanofiber membranes by several methods. Silver nanoparticles can be electrosprayed onto the surface of the nanofiber membrane, thus producing a layer of antimicrobial protection on it. The silver nanoparticles can also be blended with polymers and then electrospun. However, this can result in most of the silver nanoparticles being embedded into the polymer fibers. Growing nanoparticles on the surface of fibers is perhaps the most effective method for producing nanofibers with antimicrobial properties. This can be achieved by mixing a silver salt or sol with a polymer in a solvent and electrospinning the mixture. The collected mat containing silver ions on the fiber surface is subsequently reduced by subjecting the membrane to heat treatment, photoreduction e.g. UV irradiation or both.139,140Lala et al.141 have successfully developed silver impregnated antimicrobial filters using a number of different polymers, and have tested their antimicrobial effectiveness using two gram-negative bacterial groups: E. coli and P. aeruginosa. The membranes showed good antimicrobial activity when incubated with bacteria, consistent with other reported data in the literature. Fig. 13 shows (a) an SEM image of nanofibers collected with antimicrobial properties and (b) the TEM image of a single fiber with silver nanoparticles on its surface.
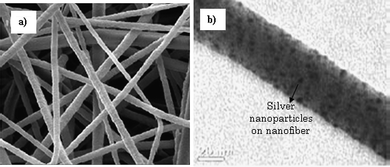 | ||
| Fig. 13 SEM images of (a) electrospun nanofibers with silver nanoparticle solution and then photoreduced by UV irradiation. (b) TEM image of a single nanofiber with silver nanoparticles on its surface. | ||
No study has so far been carried out to test the filtration performance and disinfection efficiency of the silver impregnated nanofiber membranes in a flow through system. Our group is presently studying this aspect. There is potential for the membranes to be used as an alternative to conventional disinfection methods such as chlorination and UV disinfection in water treatment or water reuse.
Besides silver nanoparticles, antibacterial polymers have also been incorporated into electrospun fibers to enhance their bactericidal properties. Commonly, quaternary ammonium salts or their derivatives or polymers with biguanide groups have been blended with polymers to provide antibacterial properties.142 Fan et al.143 have tested the bactericidal properties of alginate and carboxymethyl chitosan blended nanofibers, when (a) untreated, (b) treated with N-(2-hydroxy)-propyl-3-trimethylammonium chitosan chloride (HTCC) and (c) treated with silver nitrate. They have found that silver nitrate treated nanofibers had the best reduction rate (>99.99%) of Staphylococcus aureus, when compared with HTCC (86–90%). However, this was still much higher than the untreated nanofibers which had a bacterial reduction rate of 5–10%. In another work, quaternary ammonium salts generated on electrospun diblock copolymers by N-alkylation of tertiary amine groups were tested for their antibacterial efficiency.144 The nanofibers were found to exhibit high antibacterial characteristics with 95% of E. coli and 97% S. aureus killed within 10 min of contact with 50 mg of fibers.
13. Ion exchange
The use of nanofibers in ion exchange is still in its infancy. A study conducted using polystyrene nanofibers was able to achieve a maximum ion-exchange capacity of 3.74 mmol g−1.145 This value is comparable with commercially available ion-exchange membranes. The research suggests that ion-exchange membranes can be produced using nanofibers and there is good potential to increase its performance further.14. Membrane distillation
Membrane distillation is a filtration process that uses thermal energy as a driving force. The membrane usually separates two liquids that are of different temperatures, with transport of liquid taking place from the higher temperature side (feed) to the lower temperature side (permeate).146 Currently, membrane distillation processes employ commercially available hydrophobic microfiltration membranes. Nanofiber membranes can be used in membrane distillation directly, because the nanofiber mats generally exhibit high surface hydrophobicity. In addition, the high porosity and interconnected open pore structure characteristics of the nanofiber membrane could potentially enhance the membrane distillation process. Feng et al.147 have studied the use of poly(vinylidene fluoride) PVDF nanofiber membrane in an air-gap membrane distillation system. The preliminary results have been promising, with NaCl salt rejection rate of approximately 99.0–99.9% reported for various feed concentrations. The permeate flux of the nanofiber membrane ranged from approximately 1.5 L m−2 h−1 when the temperature difference between the liquids was 15 °C to approximately 11–12 L m−2 h−1 when the temperature difference between the liquids was 60 °C. These values are comparable with commercial membrane distillation membranes. However, with further optimization of the nanofiber membrane improved performance is possible.15. Membrane applications in bioengineering
In the bioengineering area, there is strong potential for nanofiber membranes to be used. The purification of biomolecules, such as proteins is an important step in many bioengineering processes. For example, antibody purification is a critically important step in the preparation of biopharmaceuticals and in the application of immunodiagnostics and immunotheraphy. Protein purification is commonly carried out using several techniques such as ion-exchange chromatography, gel filtration, ultrafiltration, affinity chromatography, high resolution reversed-phase chromatography, hydrophobic interaction chromatography.Of these methods, affinity chromatography and in particular affinity membranes provide a promising means of protein purification. Affinity membranes generally consist of microfiltration membranes to which specific selective–affinity ligands are attached. In addition to the usual sieving mechanisms of membrane systems, affinity membranes also allow the capture of proteins on the adsorption sites due to the immobilized ligands. Current microfiltration membranes produced by the phase-inversion or spunbonded methods tend to have low porosity.148 This reduces the surface area of the membranes available to the ligands and therefore the protein binding. Additionally, a lower porosity also implies a higher pressure drop during operation. Hence, more energy efficient membranes, with higher surface areas would greatly increase the performance and potential of the affinity membranes for biomolecule purification. Nanofiber membranes made from electrospinning offer these advantages and are of the same size range as microfiltration membranes.
As a proof of concept, regenerated cellulose nanofiber membranes have been electrospun and functionalized with protein A/G.149 Immunoglobulin G (IgG) has been used to test the capture efficiency of the nanofiber membrane. It has been found that the electrospun nanofiber affinity membrane had an IgG binding capacity of 18 μg mg−1. The nanofiber affinity membrane has been reported to have a binding capacity almost double that of a commercial affinity membrane.
16. Sensors for environmental applications
A key feature of an environmental management strategy is the detection of threats to the environment. Sensors are important tools that allow detection of contaminants in the environment. Nanofiber based sensors are gaining popularity. Below, some of the nanofiber based sensors which can be used to detect pollutants in the environment are highlighted.16.1 Sensors in liquid applications
The use of nanofiber templates for sensors in environmental applications is also gaining prominence. There are many pollutants in water that exist in small concentrations. These trace contaminants, such as pharmaceuticals, cosmetics, hormonesetc. are potential carcinogens and can cause other health problems when present in water.150 Their detection is often difficult to achieve, because they occur in parts per billion concentrations. Nanofiber based membranes are now also offering potential means of detecting these compounds in water.Molecularly imprinted nanoparticles encapsulated in polymer nanofibers have been found to be capable of detecting trace amounts of propranolol (1 ng mL−1) in tap water. Interestingly, the encapsulated nanoparticles remain accessible and are chiral selective.151 Similar work has been carried out for theophylline and 17β-estradiol imprinted nanoparticles encapsulated in polymeric nanofibers, which allowed the detection of these compounds in water.152 The advantage of introducing molecularly imprinted nanoparticles into the nanofiber membrane rather than using them on their own is that the subsequent collection or recovery of the nanoparticles from water is no longer an issue. Additionally, the nanofibers have a huge surface area compared with conventional membrane materials, so the sensitivity can be increased. Working with molecule imprinted nanofiber membrane systems opens up interesting avenues, and opportunities for sensor development in water and wastewater analysis. Consequently, it could also aid in the selective removal of various harmful pharmaceuticalse.g. endocrine disrupting compounds and contaminants in water. In cases where small concentrations of industrially important compounds are present in water, but which could result in severe problems to the environment, their detection could serve as a means of reclaiming these compounds for recycling in the manufacturing process. This will aid in limiting the increasing prevalence of trace contaminants in the environment due to the manufacturing process.
16.2 Sensors in gas applications
Air pollution as a result of industrialization is a serious problem in many developed and developing countries. Gaseous nitrogen oxides (NOx) and sulfur oxides (SOx) produced primarily due to the coal combustion process and automobiles, cause severe environmental problems such as acid rain, smog, ground level ozone, acidic aerosols etc.. These have far reaching consequences on the ecosystem and in many cases direct toxicology effects on humans. Greenhouse gases e.g.carbon dioxide (CO2), methane (CH4) etc are another important class of air pollutants, which are widely regarded to be responsible for the global warming effect. Emission caps for these gases is widely regulated in most countries and with impending carbon emission regulations to be imposed globally, the detection and measurement of these gases by industries is an important area. Nanofibers could play an important role as gas sensors for the detection of acceptable emission limits for a variety of gases.Most nanofiber membranes used as gas sensors, tend to be of ceramic materials. These ceramic nanofibers can be produced by a combination of sol–gel and electrospinning techniques.125 Gouma et al. have shown that electrospun fibers from molybdic oxide (MoO3) and tungsten oxide (WO3) could be used for detection of NO2 and NH3 in air, respectively.153,154 The nanofiber gas sensors have been found to have superior characteristics in terms of response time and sensitivity when compared with thin-film structures of the same materials. The sensors have been capable of detecting concentrations of approximately 50 ppm of NO2 and 10 ppm of NH3 using the respective electrospun nanofibers, MoO3 and WO3. Medrignac-Conanecet al.155 fabricated a mixed metal oxide nanofiber membrane consisting of molybdenum and tungsten oxides using electrospinning. They reported that this combined material could simultaneously detect a number of gases e.g.ozone, nitrogen oxide and nitrogen dioxide. It is also possible to use other combinations of metallic compounds to detect the same gas analyte, e.g. electrospun lanthanum copper oxide (La2CuO4) nanofibers, which have been used to measure NO concentration.156 The advantage of using ceramic nanofibers as gas sensors, is that they can be operated to detect gases at higher temperatures. This is useful in many industrial applications, where effluent gases released are of a high temperature.
The future gas sensors besides, having a quick response time would have to be more sensitive. This implies that they should be able to detect gases in the parts per billion range and not only the current parts per million ranges. In order to meet this goal, fiber diameter would have to reduce significantly. Electrospinning continues to be an attractive method to produce these nanofibers. Kim et al.157 have showed that TiO2–poly(vinyl acetate) composite nanofiber produced using electrospinning followed by hot-pressing and calcination, resulted in a highly sensitive NO2 gas sensor. It has been reported that the detection limit of the sensor to NO2 was estimated to be below 1 ppb. In addition, indium oxide has been found to be a highly sensitive material for the detection of several gases e.g. CO, NH3, O3, CO2, H2, NO2 and Cl2.158,159 Therefore the electrospinning of indium oxide nanofibers and/or composite materials would be extremely useful for future gas sensor applications.
17. Conclusions and prospects for electrospun nanofibers
Energy and the environment are the most important factors that influence the shape of society in the 21st century. Nanosized fibers have great advantages due to their high surface area to volume ratio, electrospun nanofibers have potential applications in the field of clean energy (solar cells, fuel cells and batteries), electronics, health (biomedical scaffolds, artificial organs), and environment (filter membranes). Current advances in electrospinning technology provide important evidence of the potential roles in energy conversion and storage as well as water, and air treatment applications. Though electrospinning has become an essential technique for generating 1D nanostructures, the research exploration is still young, but promising, in energy applications. One of the drawbacks of electrospinning is that, it has been difficult to obtain uniform nanofibers with diameters below 50 nm using electrospinning. Another drawback is the relatively low production rate. In the near future, it is likely that research efforts will be focused on engineering the electrospinning process, with the ultimate goal of producing nanofibers with diameters below 50 nm, and at a faster rate. In the long term, it is expected that vertical patterned nanofibers, an ideal morphology using electrospinning, should be possible to produce in order to achieve maximum electron transport in energy and electronic devices and controlled pore sizes for environmental filtration. This will require dedication and research collaboration within electrospinning research communities around the world. Nanofiberous membrane processes represent a key technology that provides a solution to pollution in water and air environments. It is anticipated that the future of membrane technology lies in the development of more efficient and energy saving membranes.Furthermore, the development of clean energy and environment technology will provide enormous opportunities for the creation of high value added products and associated business development. Hence, interest in ES technology is not only restricted to academic research laboratories, but also world-renowned companies such as Dupont, Johnson & Johnson, Donaldson, Teijin and, eSpin Tech etc. have been involved to produce nanofiberous products for improved performance. Ultimately, for electrospinning technology to progress further, there is an essential need to move away from high cost and/or exotic materials to low cost, non toxic or pollution free materials. Current electrospinning processes involve the use of organic solvents, which are toxic, corrosive and detrimental to the environment. This may warrant further scale up for fully-fledged industrial production. Developing the electrospinning process using water based solvents or water soluble reagents to produce nanofibers will make the process eco-friendly and open up the way for industrial production. Our research group is focused on such research under the theme of ‘green electrospinning’ by developing and implementing water soluble but still spinnable polymers.
Acknowledgements
V. Thavasi acknowledges the NUS, Singapore President Grant. G. Singh acknowledges the support from the Environment and Water Industry Grant under the Ministry of Environment and Water Resources.References
- G. D'Amato, Allergy, 2002, 57(S72), 30–33 CrossRef.
- B. Veleirinho, M. F. Rei and J. A. Lopes-da-Silva, J. Polym. Sci., Part B, 2008, 46, 460–471 CrossRef CAS.
- S. Arumuganathar, S. Irvine, J. R. McEwan and S. N. Jayasinghe, J. Appl. Polym. Sci., 2008, 107, 1215–1225 CrossRef CAS.
- R. S. Barhate, S. Sundarrajan, D. Pliszka and S. Ramakrishna, Filtr. Sep., 2008, 45, 32–35 CrossRef CAS.
- B. B. Lakshmi, P. K. Dorhout and C. R. Martin, Chem. Mater., 1997, 9, 857–862 CrossRef CAS.
- M. Law, J. Goldberger and P. Yang, Annu. Rev. Mater. Res., 2004, 34, 83–122 CrossRef CAS.
- M. S. Gudiksen and C. M. Lieber, J. Am. Chem. Soc., 2000, 122, 8801–8802 CrossRef CAS.
- X. Wang and Y. Li, J. Am. Chem. Soc., 2002, 124, 2880–2881 CrossRef CAS.
- X. Peng, U. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Allvisatos, Nature, 2000, 404, 59–61 CrossRef CAS.
- N. I. Kovtyukhova, B. R. Martin, J. K. N. Mbindyo, T. E. Mallouk, M. Cabassi and T. S. Mayer, Mater. Sci. Eng., C, 2002, C19, 255–262 CrossRef CAS.
- J. Doshi and D. H. Reneker, J. Electrost., 1995, 35, 151–160 CrossRef CAS.
- M. B. Bazbouz and G. K. Stylios, J. Appl. Polym. Sci., 2008, 107, 3023–3032 CrossRef CAS.
- M. B. Bazbouz and G. K. Stylios, Eur. Polym. J., 2007, 44, 1–12.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3, 232–238 CrossRef CAS.
- D. Li, Y. Wang and Y. Xia, Nano Lett., 2003, 3, 1167–1171 CrossRef CAS.
- B. Sundaray, V. Subramanian, T. S. Natarajan, R.-Z. Xiang, C.-C. Chang and W.-S. Fann, Appl. Phys. Lett., 2004, 84, 1222–1224 CrossRef CAS.
- B. Sundaray, V. Subramanian, T. S. Natarajan and K. Krishnamurthy, Appl. Phys. Lett., 2006, 88, 143111–143113 CrossRef.
- Y. Z. Zhang, J. Venugopal, Z. M. Huang, C. T. Lim and S. Ramakrishna, Polymer, 2006, 47, 2911–2917 CrossRef CAS.
- Z. Sun, E. Zussman, A. L. Yarin, J. H. Wendorff and A. Greiner, Adv. Mater., 2003, 15, 1929–1932 CrossRef CAS.
- A. Babel, D. Li, Y. Xia and S. A. Jenekhe, Macromolecules, 2005, 38, 4705–4711 CrossRef CAS.
- M. Wei, J. Lee, B. Kang and J. Mead, Macromol. Rapid Commun., 2005, 26, 1127–1132 CrossRef CAS.
- B. O'Regan and M. Graetzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- T. Aernouts, P. Vanlaeke, W. Geens, J. Poortmans, P. Heremans, S. Borghs, R. Mertens, R. Andriessen and L. Leenders, Thin Solid Films, 2004, 451–452, 22–25 CrossRef.
- K. Srikanth, M. M. Rahman, H. Tanaka, K. M. Krishna, T. Soga, M. K. Mishra, T. Jimbo and M. Umeno, Sol. Energy Mater. Sol. Cells, 2001, 65, 171–177 CrossRef CAS.
- J. Hagen, W. Schaffrath, P. Otschik, R. Fink, A. Bacher, H.-W. Schmidt and D. Haarer, Synth. Met., 1997, 89, 215–220 CrossRef CAS.
- D. Zhang, S. Ito, Y. Wada, T. Kitamura and S. Yanagida, Chem. Lett., 2001, 1042–1043 CrossRef CAS.
- B. O. Aduda, P. Ravirajan, K. L. Choy and J. Nelson, Int. J. Photoenergy, 2004, 6, 141–147 CrossRef CAS.
- M. G. Nolan, M. E. Pemble, D. W. Sheel and H. M. Yates, Thin Solid Films, 2006, 515, 1956–1962 CrossRef CAS.
- K. Onozuka, B. Ding, Y. Tsuge, T. Naka, M. Yamazaki, S. Sugi, S. Ohno, M. Yoshikawa and S. Shiratori, Nanotechnology, 2006, 17, 1026–1031 CrossRef CAS.
- E. Davies, A. Lowe, M. Sterns, K. Fujihara and S. Ramakrishna, J. Am. Ceram. Soc., 2008, 91, 1115–1120 CrossRef CAS.
- M. Y. Song, D. K. Kim, S. M. Jo and D. Y. Kim, Synth. Met., 2005, 155, 635–638 CrossRef CAS.
- S. M. Jo, M. Y. Song, Y. R. Ahn, C. R. Park and D. Y. Kim, J. Macromol. Sci., Part A, 2005, A42, 1529–1540 CAS.
- M. Y. Song, D. K. Kim, K. J. Ihn, S. M. Jo and D. Y. Kim, Nanotechnology, 2004, 15, 1861–1865 CrossRef CAS.
- K. Fujihara, A. Kumar, R. Jose, S. Ramakrishna and S. Uchida, Nanotechnology, 2007, 18, 365701–365705 CrossRef.
- K. H. Ko, Y. C. Lee and Y. J. Jung, J. Colloid Interface Sci., 2005, 283, 482–487 CrossRef CAS.
- W. Li, A. I. Frenkel, J. C. Woicik, C. Ni and S. I. Shah, Phys. Rev. B, 2005, 72, 155311–155316 CrossRef.
- M. Y. El Zayat, A. O. Saed and M. S. El-Dessouki, Sol. Energy Mater. Sol. Cells, 2002, 71, 27–39 CrossRef CAS.
- J. H. Schon, C. Kloc, E. Bucher and B. Batlogg, Nature, 2000, 403, 408–410 CrossRef CAS.
- Y. Diamant, S. Chappel, S. G. Chen, O. Melamed and A. Zaban, Coord. Chem. Rev., 2004, 248, 1271–1276 CrossRef CAS.
- V. Tomer, R. Teye-Mensah, J. C. Tokash, N. Stojilovic, W. Kataphinan, E. A. Evans, G. G. Chase, R. D. Ramsier, D. J. Smith and D. H. Reneker, Sol. Energy Mater. Sol. Cells, 2005, 85, 477–488 CrossRef CAS.
- S. Berson, R. De Bettignies, S. Bailly and S. Guillerez, Adv. Funct. Mater., 2007, 17, 1377–1384 CrossRef CAS.
- X. Lu, Q. Zhao, X. Liu, D. Wang, W. Zhang, C. Wang and Y. Wei, Macromol. Rapid Commun., 2006, 27, 430–434 CrossRef CAS.
- I. S. Chronakis, S. Grapenson and A. Jakob, Polymer, 2006, 47, 1597–1603 CrossRef CAS.
- S. Nair, S. Natarajan and S. H. Kim, Macromol. Rapid Commun., 2005, 26, 1599–1603 CrossRef CAS.
- M. Y. Lo, C. Zhen, M. Lauters, G. E. Jabbour and A. Sellinger, J. Am. Chem. Soc., 2007, 129, 5808–5809 CrossRef CAS.
- N. Kudo, Y. Shimazaki, H. Ohkita, M. Ohoka and S. Ito, Sol. Energy Mater. Sol. Cells, 2007, 91, 1243–1247 CrossRef CAS.
- C. Wang, E. Yan, Z. Huang, Q. Zhao and Y. Xin, Macromol. Rapid Commun., 2007, 28, 205–209 CrossRef CAS.
- D. C. Olson, J. Piris, R. T. Collins, S. E. Shaheen and D. S. Ginley, Thin Solid Films, 2005, 496, 26–29.
- B. Pradhan, S. K. Batabyal and A. J. Pal, Sol. Energy Mater. Sol. Cells, 2007, 91, 769–773 CrossRef CAS.
- P. Ravirajan, A. M. Peiro, M. K. Nazeeruddin, M. Graetzel, D. D. C. Bradley, J. R. Durrant and J. Nelson, J. Phys. Chem. B, 2006, 110, 7635–7639 CrossRef CAS.
- G. T. Wang, A. A. Talin, D. J. Werder, J. R. Creighton, E. Lai, R. J. Anderson and I. Arslan, Nanotechnology, 2006, 17, 5773–5780 CrossRef CAS.
- S. Kolodinski, J. H. Werner, T. Wittchen and H. J. Queisser, Appl. Phys. Lett., 1993, 63, 2405–2407 CrossRef CAS.
- R. D. Schaller, M. Sykora, J. M. Pietryga and V. I. Klimov, Nano Lett., 2006, 6, 424–429 CrossRef CAS.
- N. C. Greenham, X. Peng and A. P. Alivisatos, Synth. Met., 1997, 84, 545–546 CrossRef CAS.
- W. U. Huynh, J. J. Dittmer and A. P. Alivisatos, Science, 2002, 295, 2425–2427 CrossRef CAS.
- W. U. Huynh, X. Peng and A. P. Alivisatos, Adv. Mater., 1999, 11, 923–927 CrossRef CAS.
- C.-M. Chen, M. Chen, H.-W. Yu, S.-C. Lu and C.-F. Chen, Jpn. J. Appl. Phys., Part 1, 2008, 47, 2324–2329 CrossRef CAS.
- G. K. Chandler, J. D. Genders and D. Pletcher, Platinum Met. Rev., 1997, 41, 54–63 CAS.
- X. Ren, M. S. Wilson and S. Gottesfeld, J. Electrochem. Soc., 1996, 143, L12–L15 CAS.
- S. Surampudi, S. R. Narayanan, E. Vamos, H. Frank, G. Halpert, A. LaConti, J. Kosek, G. K. S. Prakash and G. A. Olah, J. Power Sources, 1994, 47, 377–385 CrossRef CAS.
- K. Wang, H. A. Gasteiger, N. M. Markovic and P. N. Ross, Jr., Electrochim. Acta, 1996, 41, 2587–2593 CrossRef CAS.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS.
- F. Zaragoza-Martin, D. Sopena-Escario, E. Morallon and C. Salinas-Martinez de Lecea, J. Power Sources, 2007, 171, 302–309 CrossRef CAS.
- W. D. King, J. D. Corn, O. J. Murphy, D. L. Boxall, E. A. Kenik, K. C. Kwiatkowski, S. R. Stock and C. M. Lukehart, J. Phys. Chem. B, 2003, 107, 5467–5474 CrossRef.
- E. S. Steigerwalt, G. A. Deluga and C. M. Lukehart, J. Phys. Chem. B, 2002, 106, 760–766 CrossRef CAS.
- J. S. Lee, K. I. Han, S. O. Park, H. N. Kim and H. Kim, Electrochim. Acta, 2004, 50, 807–810 CrossRef CAS.
- E. S. Steigerwalt, G. A. Deluga, D. E. Cliffel and C. M. Lukehart, J. Phys. Chem. B, 2001, 105, 8097–8101 CrossRef.
- F.-J. Liu, L.-M. Huang, T.-C. Wen and A. Gopalan, Synth. Met., 2007, 157, 651–658 CrossRef CAS.
- Z. Chen, L. Xu, W. Li, M. Waje and Y. Yan, Nanotechnology, 2006, 17, 5254–5259 CrossRef CAS.
- H. Gharibi, M. Zhiani, A. A. Entezami, R. A. Mirzaie, M. Kheirmand and K. Kakaei, J. Power Sources, 2006, 155, 138–144 CAS.
- Z. Liu, X. Lin, J. Y. Lee, W. Zhang, M. Han and L. M. Gan, Langmuir, 2002, 18, 4054–4060 CrossRef CAS.
- M. Gangeri, G. Centi, A. La Malfa, S. Perathoner, R. Vieira, C. Pham-Huu and M. J. Ledoux, Catal. Today, 2005, 102–103, 50–57 CrossRef CAS.
- W. Li, C. Liang, W. Zhou, J. Qiu, Z. Zhou, G. Sun and Q. Xin, J. Phys. Chem. B, 2003, 107, 6292–6299 CrossRef CAS.
- G. Che, B. B. Lakshmi, C. R. Martin and E. R. Fisher, Langmuir, 1999, 15, 750–758 CrossRef CAS.
- C. A. Bessel, K. Laubernds, N. M. Rodriguez and R. T. K. Baker, J. Phys. Chem. B, 2001, 105, 1115–1118 CrossRef CAS.
- E. S. Steigerwalt, G. A. Deluga and C. M. Lukehart, J. Nanosci. Nanotechnol., 2003, 3, 247–251 CrossRef CAS.
- V. Hacker, E. Wallnoefer, W. Baumgartner, T. Schaffer, J. O. Besenhard, H. Schroettner and M. Schmied, Electrochem. Commun., 2005, 7, 377–382 CrossRef CAS.
- Y. You, S. W. Lee, S. J. Lee and W. H. Park, Mater. Lett., 2006, 60, 1331–1333 CrossRef CAS.
- S. O. Han, W. K. Son, D. Cho, J. H. Youk and W. H. Park, Polym. Degrad. Stab., 2004, 86, 257–262 CrossRef CAS.
- D. Li, J. T. McCann, Y. Xia and M. Marquez, J. Am. Ceram. Soc., 2006, 89, 1861–1869 CrossRef CAS.
- J.-H. He, Y. Liu, L. Xu and J.-Y. Yu, Chaos, Solitons Fractals, 2006, 32, 1096–1100.
- J.-H. He, Y.-Q. Wan and L. Xu, Chaos, Solitons Fractals, 2006, 33, 26–37.
- R. S. Barhate, C. K. Loong and S. Ramakrishna, J. Membr. Sci., 2006, 283, 209–218 CrossRef CAS.
- F. Yuan and H. Ryu, Nanotechnology, 2004, 15, S596–S602 CrossRef CAS.
- E. Yasuda, T. Enami, N. Hoteida, L. J. Lanticse-Diaz, Y. Tanabe and T. Akatsu, Mater. Sci. Eng., B, 2008, 148, 7–12 CrossRef CAS.
- A. C. Patel, S. Li, J.-M. Yuan and Y. Wei, Nano Lett., 2006, 6, 1042–1046 CrossRef CAS.
- D. Lueking Angela, L. Pan, L. Narayanan Deepa and E. B. Clifford Caroline, J. Phys. Chem. B, 2005, 109, 12710–12717 CrossRef CAS.
- J. P. Maranchi, O. I. Velikokhatnyi, M. K. Datta, I.-S. Kim and P. N. Kumta, Mater. Eng., 2005, 28, 667–711 CAS.
- J. Fan and P. S. Fedkiw, J. Power Sources, 1998, 72, 165–173 CrossRef CAS.
- N. J. Dudney, Mater. Sci. Eng., B, 2005, B116, 245–249 CrossRef CAS.
- A. Du Pasquier, I. Plitz, S. Menocal and G. Amatucci, J. Power Sources, 2003, 115, 171–178 CrossRef CAS.
- Y. Gu, D. Chen and X. Jiao, J. Phys. Chem. B, 2005, 109, 17901–17906 CrossRef CAS.
- C. Shao, N. Yu, Y. Liu and R. Mu, J. Phys. Chem. Solids, 2006, 67, 1423–1426 CrossRef CAS.
- L. Wang, Y. Yu, P.-C. Chen and C.-H. Chen, Scr. Mater., 2007, 58, 405–408.
- C. Kim, K. S. Yang, M. Kojima, K. Yoshida, Y. J. Kim, Y. A. Kim and M. Endo, Adv. Funct. Mater., 2006, 16, 2393–2397 CrossRef CAS.
- C. Kim, J. Power Sources, 2005, 142, 382–388 CrossRef CAS.
- F. Pico, J. Ibanez, M. A. Lillo-Rodenas, A. Linares-Solano, R. M. Rojas, J. M. Amarilla and J. M. Rojo, J. Power Sources, 2008, 176, 417–425 CrossRef CAS.
- S. R. Sivakkumar, W. J. Kim, J.-A. Choi, D. R. MacFarlane, M. Forsyth and D.-W. Kim, J. Power Sources, 2007, 171, 1062–1068 CrossRef CAS.
- Y. Luo, Z. Hou, J. Gao, D. Jin and X. Zheng, Mater. Sci. Eng., B, 2007, 140, 123–127 CrossRef CAS.
- K. Suzuki, Y. Kuroki, T. Okamoto and M. Takata, Adv. Technol. Mater., Mater. Process. J., 2007, 9, 77–80 Search PubMed.
- Y. Zhang, J. Yang, Q. Li and X. Cao, J. Cryst. Growth, 2007, 308, 180–184 CrossRef CAS.
- C.-C. Kuo, C.-H. Lin and W.-C. Chen, Macromolecules, 2007, 40, 6959–6966 CrossRef CAS.
- D. Li, G. Ouyang, J. T. McCann and Y. Xia, Nano Lett., 2005, 5, 913–916 CrossRef CAS.
- D. Li, Y. Wang and Y. Xia, Adv. Mater., 2004, 16, 361–366 CrossRef CAS.
- L. Dai, X. L. Chen, J. K. Jian, W. J. Wang, T. Zhou and B. Q. Hu, Appl. Phys. A: Mater. Sci. Process., 2003, 76, 625–627 CrossRef CAS.
- X. D. Bai, C. Y. Zhi and E. G. Wang, J. Nanosci. Nanotechnol., 2001, 1, 55–58 CrossRef CAS.
- X. D. Bai, E. G. Wang, J. Yu and H. Yang, Appl. Phys. Lett., 2000, 77, 67–69 CrossRef CAS.
- E. Atanassova, D. Spassov and A. Paskaleva, Microelectron. Reliab., 2007, 47, 2088–2093 CrossRef CAS.
- J.-P. Manceau, S. Bruyere, S. Jeannot, A. Sylvestre and P. Gonon, IEEE Trans. Device Mater. Reliab., 2007, 7, 315–323 CrossRef CAS.
- Y. Ohtera, H. Ohkubo, K. Miura, T. Sato, T. Kawashima and S. Kawakami, Opt. Eng., 2004, 43, 1022–1029 CrossRef CAS.
- N. Dharmaraj, H. C. Park, C. H. Kim, P. Viswanathamurthi and H. Y. Kim, Mater. Res. Bull., 2006, 41, 612–619 CrossRef CAS.
- A. Kumar, R. Jose, K. Fujihara, J. Wang and S. Ramakrishna, Chem. Mater., 2007, 19, 6536–6542 CrossRef CAS.
- M. R. Wiesner and S. Chellam, Environ. Sci. Technol., 1999, 33, 360A–366A CAS.
- J. A. Howell, Desalination, 2004, 162, 1–11 CrossRef CAS.
- S. Kaur, R. Gopal, W. J. Ng, S. Ramakrishna and T. Matsuura, MRS Bull., 2008, 33, 21–26 CAS.
- R. S. Barhate and S. Ramakrishna, J. Membr. Sci., 2007, 296, 1–8 CrossRef CAS.
- G. W. Selling and K. K. Woods, Cereal Chem., 2008, 85, 202–206 CrossRef CAS.
- J.-S. Im, S. J. Park and Y.-S. Lee, Diffus. Defect Data, Pt. B: Solid State Phenom., 2008, 135, 81–84 Search PubMed.
- V. Kalayci, M. Ouyang and K. Graham, Filtration, 2006, 6, 286–296 Search PubMed.
- S. Arumuganathar and S. N. Jayasinghe, Biomed. Mater., 2007, 2, 189–195 Search PubMed.
- M. V. Jose, B. W. Steinert, V. Thomas, D. R. Dean, M. A. Abdalla, G. Price and G. M. Janowski, Polymer, 2007, 48, 1096–1104 CrossRef CAS.
- H. Schreuder-Gibson, P. Gibson, L. Wadsworth, S. Hemphill and J. Vontorcik, Adv. Filtr. Sep. Technol., 2002, 15, 525–537 Search PubMed.
- Z.-M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- R. Ramaseshan, S. Sundarrajan, Y. Liu, R. S. Barhate, N. L. Lala and S. Ramakrishna, Nanotechnology, 2006, 17, 2947–2953 CrossRef CAS.
- R. Ramaseshan, S. Sundarrajan, R. Jose and S. Ramakrishna, J. Appl. Phys., 2007, 102, 111101–111117 CrossRef.
- S. D. McCullen, D. R. Stevens, W. A. Roberts, L. I. Clarke, S. H. Bernacki, R. E. Gorga and E. G. Loboa, Int. J. Nanomed., 2007, 2, 253–263 CAS.
- J.-S. Jeong, S.-J. Park, Y.-H. Shin, Y.-J. Jung, P. S. Alegaonkar and J.-B. Yoo, Diffus. Defect Data, Pt. B: Solid State Phenom., 2007, 124–126, 1125–1128 Search PubMed.
- C. P. Barnes, C. W. Pemble, D. D. Brand, D. G. Simpson and G. L. Bowlin, Tissue Eng., 2007, 13, 1593–1605 CrossRef CAS.
- S. Kaur, Z. Ma, R. Gopal, G. Singh, S. Ramakrishna and T. Matsuura, Langmuir, 2007, 23, 13085–13092 CrossRef CAS.
- R. Gopal, S. Kaur, Z. Ma, C. Chan, S. Ramakrishna and T. Matsuura, J. Membr. Sci., 2006, 281, 581–586 CrossRef CAS.
- C. S. Ki, E. H. Gang, I. C. Um and Y. H. Park, J. Membr. Sci., 2007, 302, 20–26 CrossRef CAS.
- G. Hota, B. R. Kumar, W. J. Ng and S. Ramakrishna, J. Mater. Sci., 2008, 43, 212–217 CrossRef CAS.
- S. Kaur, M. Kotaki, Z. Ma, R. Gopal, S. Ramakrishna and S. C. Ng, Int. J. Nanosci., 2006, 5, 1–11 CrossRef CAS.
- S. C. B. Myneni, J. T. Brown, G. A. Martinez and W. Meyer-Llse, Science, 1999, 286, 1335–1337 CrossRef CAS.
- X. Zhang, A. J. Du, P. Lee, D. D. Sun and J. O. Leckie, J. Membr. Sci., 2008, 313, 44–51 CrossRef CAS.
- K. Yoon, K. Kim, X. Wang, D. Fang, B. S. Hsiao and B. Chu, Polymer, 2006, 47, 2434–2441 CrossRef CAS.
- Z. Ma, M. Kotaki and S. Ramakrishna, J. Membr. Sci., 2006, 272, 179–187 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS.
- K. H. Hong, J. L. Park, I. H. Sul, J. H. Youk and T. J. Kang, J. Polym. Sci., Part B, 2006, 44, 2468–2474 CrossRef CAS.
- H. K. Lee, E. H. Jeong, C. K. Baek and J. H. Youk, Mater. Lett., 2005, 59, 2977–2980 CrossRef CAS.
- N. L. Lala, R. Ramaseshan, B. Li, S. Sundarrajan, R. S. Barhate, Y.-J. Liu and S. Ramakrishna, Biotechnol. Bioeng., 2007, 97, 1357–1365 CrossRef CAS.
- M. Ignatova, N. Manolova and I. Rashkov, Eur. Polym. J., 2007, 43, 1112–1122 CrossRef CAS.
- L. Fan, Y. Du, B. Zhang, J. Yang, J. Zhou and J. F. Kennedy, Carbohydr. Polym., 2006, 65, 447–452 CrossRef CAS.
- G.-D. Fu, F. Yao, Z. Li and X. Li, J. Mater. Chem., 2008, 18, 859–867 RSC.
- H. An, C. Shin and G. G. Chase, J. Membr. Sci., 2006, 283, 84–87 CrossRef CAS.
- E. Curcio and E. Drioli, Sep. Purif. Rev., 2005, 34, 35–86 CrossRef CAS.
- C. Feng, K. C. Khulbe, T. Matsuura, R. Gopal, S. Kaur, S. Ramakrishna and M. Khayet, J. Membr. Sci., 2008, 311, 1–6 CrossRef CAS.
- X. Lu, W. Zhang, Q. Zhao, L. Wang and C. Wang, e-Polymers, 2006 Search PubMed.
- A.-f. Che, Y.-f. Yang, L.-s. Wan, J. Wu and Z.-k. Xu, Chem. Res. Chinese Univ., 2006, 22, 390–393 Search PubMed.
- H. F. Kraybill, Bull. N.Y. Acad. Med., 1978, 54, 413–427 Search PubMed.
- K. Yoshimatsu, L. Ye, J. Lindberg and I. S. Chronakis, Biosens. Bioelectron., 2008, 23, 1208–1215 CrossRef CAS.
- I. S. Chronakis, A. Jakob, B. Hagstroem and L. Ye, Langmuir, 2006, 22, 8960–8965 CrossRef CAS.
- P. I. Gouma, Science, 2003, 5, 147–154 CAS.
- K. M. Sawicka, A. K. Prasad and P. I. Gouma, Sensor Lett., 2005, 3, 31–35 Search PubMed.
- O. Merdrignac-Conanec and P. T. Moseley, J. Mater. Chem., 2002, 12, 1779–1781 RSC.
- K. Sawicka, P. Gouma and S. Simon, Proc. Electrochem. Soc., 2004, 2004–08, 354–358 CAS.
- I.-D. Kim, A. Rothschild, B. H. Lee, D. Y. Kim, S. M. Jo and H. L. Tuller, Nano Lett., 2006, 6, 2009–2013 CrossRef CAS.
- J. Lao, J. Huang, D. Wang and Z. Ren, Adv. Mater., 2004, 16, 65–69 CrossRef CAS.
- C.-Y. Kuo, S.-Y. Lu and T.-Y. Wei, J. Cryst. Growth, 2005, 285, 400–407 CrossRef CAS.
- R. Zhu, C.-Y. Jiang, X.-Z. Liu, B. Liu, A. Kumar and S. Ramakrishna, Appl. Phys. Lett., 2008, 93, 013101–013103 CrossRef.
Footnote |
| † Equal contributors. |
| This journal is © The Royal Society of Chemistry 2008 |
