Direct observation of change in the molecular structure of benzyl (Z,Z)-muconate during photoisomerization in the solid state†
Daisuke
Furukawa
,
Seiya
Kobatake
and
Akikazu
Matsumoto
*
Department of Applied Chemistry, Graduate School of Engineering, Osaka City University, Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: matsumoto@a-chem.eng.osaka-cu.ac.jp; Fax: +81-6-6605-2981
First published on 16th November 2007
Abstract
For the solid-state photoisomerization of benzyl (Z,Z)-muconate to the corresponding (E,E)-muconate, the direct observation of a change in the crystal structure has revealed that the isomerization occurs by a topochemical reaction process according to a bicycle-pedal model and is finally accompanied by a phase transition to a stable crystal structure.
Organic reactions performed in the solid state have many intrinsic features and merits for synthetic and materials chemistry because of the extremely high selectivity under crystalline-lattice control.1,2 A limited number of isomerizations of olefins between E and Z forms in the crystalline state have been reported because of the difficulty in the inevitable change in the size and shape of the space occupied by the substituents of a double bond.3 The isomerization of polyenes is an important photochemical process in biological systems, and a bicycle-pedal model as the volume-conserving reaction mechanism was first pointed out by Warshel in 1976.4 Later, Liu et al.5 proposed the hula-twist process to explain the results of the picosecond time-resolved kinetics of the reaction in the visual pigment rhodopsin. This model has been applied to not only photoisomerization in a biosystem, but also various isomerization reactions in confined media such as viscous fluid, rigid matrix, organic glass, and organic crystals, as well as the isomerization of olefins with bulky substituents.6,7 Reactions proceeding according to the bicycle-pedal8,9 and hula-twist models,5–7 as well as reactions including crankshaft motion,10 require only a small change in the molecular shape, differing from the conventional one-bond flip motion, which is usually observed during many reactions in solution.
Several years ago, we found that the isomerization of n-butylammonium (Z,Z)-muconate produces the corresponding E,E isomer in the crystalline state under photoirradiation.11 This solid-state photoisomerization has some characteristics: no formation of E,Z isomer and one-way reaction from the Z,Z to E,E form. In general, it is well-known that unsaturated compounds such as olefins , polyenes, and azo compounds undergo reversible one-bond photoisomerization12 to form a mixture of isomers. In our preliminary studies, we pointed out the possibility that the topochemical isomerization of the muconic derivatives in the solid state follows the bicycle-pedal reaction mechanism,2 but the molecular dynamics and mechanism of the reaction have not yet been clarified. Recently, Saltiel et al.9 and Liu et al.6 have independently discussed volume-conserving reaction mechanisms for the isomerization of 1,4-diphenyl-1,3-butadienes, 1,6-disubstituted hexatrienes13 and muconates in the crystalline state and the other confined media. In the present study, we have revealed the reaction mechanism of the solid-state photoisomerization of benzyl (Z,Z)-muconate (1) to benzyl (E,E)-muconate (2) by the direct observation of a change in the single crystal structure during the photoisomerization in the solid state.
The single crystal structures of 1 and 2, which were recrystallized from n-hexane and ethanol, respectively, are shown in Fig. 1.‡ The crystallographic parameters are summarized in Table 1. The monitoring of the change in the single-crystal structure of 1 was successfully carried out during the photoisomerization using a band path filter (UV-D36B) to irradiate light of wavelength longer than 300 nm, corresponding to the absorption edge of 1. The crystal structure of 1 after UV light irradiation was solved using the structure before photoirradiation. The space group was the same as the crystal before photoirradiation. They have crystallographically imposed inversion symmetry. As shown in Table 1, the crystal of 1 after the 27 h photoirradiation has cell volume and density values (V = 858.8 Å3, ρ = 1.247 g cm−3) identical to those of the crystal of 2 prepared by recrystallization (V = 859.5 Å3, ρ = 1.246 g cm−3), but the packing mode of the molecules in the crystals is different; the crystal of 1 has the space group of P21/c while the crystal of 2 has P21. For the crystal structure after photoirradiation, a disordered structure was observed around the diene moiety, as shown by the ORTEP drawing in Fig. 2(b). This disordered structure contains both 1 and 2 as the molecular structures. Fig. 2(c) shows the separated structures. The conversion after the photoirradiation was estimated to be 35% from the site occupancy factor of the produced 2 in the crystal. Both the Z,Z and E,E isomers included in the crystal after photoirradiation in Fig. 2(c) have similar molecular shapes and planar conformations. The direct observation of a crystal structure during the reaction has revealed that the diene moiety changes its geometric structure of the Z,Z isomer into the E,E isomer consistent with the bicycle-pedal model (Scheme 1). This is evidenced by the fact that the s-cis conformation of the reactant 1 is maintained in the product 2. The change in the volume and shape of the reacting molecule is small as expected for a reaction proceeding via the bicycle-pedal mechanism. In fact, no large space is seen nearby to the diene moiety in the crystals in Fig. 1(c). Bicycle-pedal motion needs a smaller space in the solid, and a direct relationship is not observed between the bicycle-pedal process and void space14 for the photoisomerization of muconates.
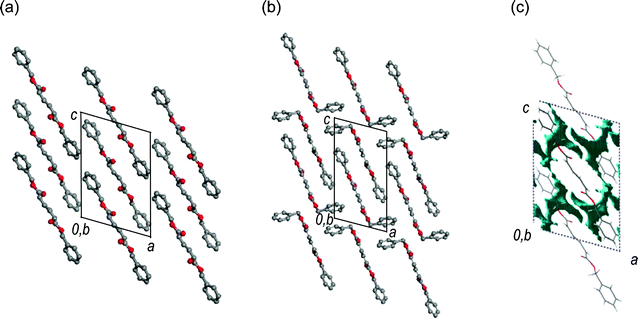 | ||
| Fig. 1 Single crystal structures of (a) 1 and (b) 2. Gray and red circles represent carbon and oxygen atoms, respectively. Hydrogen atoms are omitted for clarity. (c) Drawing of void space (blue) in the unit cell for 1. | ||
| 1 | 1 (after UV irrad.)a | 2 | |
|---|---|---|---|
| a After the photoirradiation of 1 for 27 h at room temperature using an ultra-high pressure mercury lamp (500 W), a band path filter UV-D36B and an infrared absorption filter IRA-25S. The conversion to 2 was 35%. | |||
| Space group | P21/c | P21/c | P21 |
| a/Å | 10.332(4) | 10.328(6) | 9.167(3) |
| b/Å | 5.682(2) | 5.765(4) | 5.7038(11) |
| c/Å | 14.791(7) | 15.005(9) | 17.002(5) |
| β/° | 105.86(4) | 106.00(3) | 104.803(4) |
| V/Å3 | 835.2(6) | 858.8(9) | 859.5(4) |
| Z | 2 | 2 | 2 |
| ρ calc/g cm−3 | 1.282 | 1.247 | 1.246 |
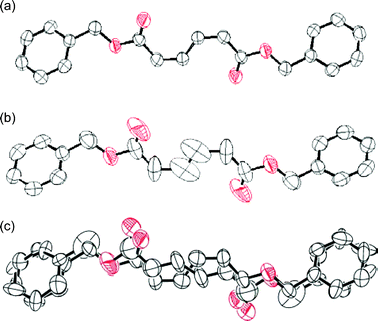 | ||
| Fig. 2 ORTEP drawings of (a) 1 crystals as the initial molecular structure, (b) disordered structure after a 27 h photoirradiation, and (c) the structure separated into 1 and 2 with a site occupancy factor of 35% for 2. The hydrogen atoms are omitted for clarity. The thermal ellipsoids are plotted at the 50% probability level. | ||
 | ||
| Scheme 1 Solid-state photoisomerization of 1via the bicycle-pedal model. The phenyl ring is omitted for clarity. | ||
The molecular conformation observed during the isomerization of 1 to 2 was compared with the geometrical parameters determined for the 1 and 2 molecules in each crystal, as well as the structures optimized by DFT calculation (See Table S2 in the ESI for detailed results† ). The geometrical parameters include a small change in the molecular structure of 1 during the photoisomerization from the structure of Fig. 2(a) to that of Fig. 2(c). The small geometry difference between the reactant and the product minimizes crystal lattice strain as the reaction progresses. The topochemical isomerization of 1 is accompanied by the expansion of the lattice lengths and the cell volume, but without changing the space group of the crystals, as shown in Table 1. Eventually, a structural strain is included in the molecules of 2 as the primary photoproduct, because the produced 2 molecules exist in the crystal lattice of the 1 molecules. Furthermore, a structural change in the crystals of 1 was observed at a higher conversion based on a powder X-ray diffraction analysis . The conversion of 1 to 2 achieved 97.5%, which was determined by NMR spectroscopy, after 120 h photoirradiation. X-Ray diffraction lines after the reaction agreed well with those for the recrystallized 2 (Fig. 3). The molecular conformation of 2 includes an asymmetric structure with a bent benzyl moiety, as shown in Fig. 1(b), being different from the symmetric conformation of 1 in the crystals of 1, and also 2 in the co-crystals formed during photoirradiation. These results indicate that a phase transition occurs at the final stage of the isomerization process. The molecules of 2 change their conformation in the solid state using the void space around the benzyl moiety shown in Fig. 1(c) during the phase transition. Namely, the primary E,E isomer produced on photoirradiation has a molecular length similar to that of the original Z,Z isomer 1 at the initial stage of the reaction, but the cell length increased along the directions of the b- and c-axes during the reaction, leading to an increase in the total volume of the crystals. Drastic changes in molecular conformation and packing structure (the slide of molecules resulting in further increase in the c-axis length) occur during the crystal-to-crystal transition to the stable crystal structure of 2, accompanying a phase separation as shown in Fig. 3(e).
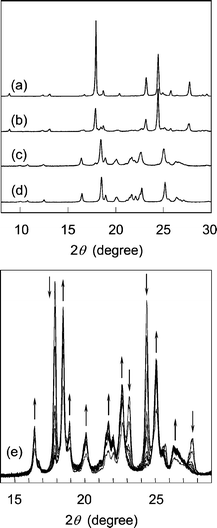 | ||
| Fig. 3 Powder X-ray diffraction profile of 1 at different conversions during the photoisomerization at room temperature. (a) 10.6%, (b) 34.0%, (c) 97.5%, and (d) 2 recrystallized from a solution. (e) Change in the profile during the photoirradiation for 20 to 120 h at 10 h interval. For the photoirradiation, an ultra-high pressure mercury lamp (500 W) and an infrared absorption filter IRA-25S were used. | ||
In conclusion, an X-ray single-crystal structure analysis has revealed that the isomerization of 1 proceeds according to a bicycle-pedal model. The isomerization occurs via a topochemical reaction process which does not require the significant movement of atoms. The reaction finally causes a phase transition to crystals of 2 with a molecular packing structure identical to that of the recrystallized 2. At the same time, the space group changes from P21/c to P21 during the phase transition. The void space included in the crystals plays an important role in the phase transition rather than in the isomerization.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Area No. 432) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. We thank Prof. Norimitsu Tohnai, Osaka University, for his kind assistance for the calculation of a void space.
Notes and references
- Reviews: V. Ramamurthy and K. Venkatesan, Chem. Rev., 1987, 87, 433 Search PubMed; K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025 CrossRef CAS; L. R. MacGillivray, G. S. Papaefstathiou, T. Friščić, D. B. Varshney and T. D. Hamilton, Top. Curr. Chem., 2004, 248, 201 CrossRef CAS; G. Kaupp, Top. Curr. Chem., 2005, 254, 95; D. Braga and F. Grepioni, Chem. Commun., 2005, 3635 CAS; J. Harada and K. Ogawa, Top. Stereochem., 2006, 25, 31 RSC; T. A. V. Khuong, J. E. Nunez, C. E. Godinez and M. A. Garcia-Garibay, Acc. Chem. Res., 2006, 39, 413 Search PubMed.
- A. Matsumoto, Top. Curr. Chem., 2005, 254, 263 CAS.
- S. M. Alfimov and V. F. Razumov, Mol. Cryst. Liq. Cryst., 1978, 49, 95 CrossRef; K. Kinbara, A. Kai, Y. Maekawa, Y. Hashimoto, S. Naruse, M. Hasegawa and K. Saigo, J. Chem. Soc., Perkin Trans. 2, 1996, 247 RSC; G. Kaupp, Photochem. Photobiol., 2002, 76, 590 CrossRef CAS; J. N. Moorthy, P. Venkatakrishnan, G. Savitha and R. G. Weiss, Photochem. Photobiol. Sci., 2006, 5, 903 RSC; A. Natarajan, J. T. Mague, K. Venkatesan, T. Arai and V. Ramamurthy, J. Org. Chem., 2006, 71, 1055 CrossRef CAS.
- A. Warshel, Nature, 1976, 260, 679 CAS.
- R. S. H. Liu and A. E. Asato, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 259 CAS; R. S. H. Liu and G. S. Hammond, Acc. Chem. Res., 2005, 38, 396 CrossRef CAS.
- R. S. H. Liu, Photochem. Photobiol., 2002, 76, 580 CrossRef CAS; R. S. H. Liu, L.-Y. Yang and J. Liu, Photochem. Photobiol., 2007, 83, 2 CAS.
- S. Schieffer, J. Pescatore, R. Ulsh and R. S. H. Liu, Chem. Commun., 2004, 2680 RSC; L.-Y. Yang, R. S. H. Liu, K. J. Boarman, N. L. Wendt and J. Liu, J. Am. Chem. Soc., 2005, 127, 2404 CrossRef CAS; L.-Y. Yang, M. Harigai, Y. Imamoto, M. Kataoka, T.-I. Ho, E. Andrioukhina, O. Federova, S. Shevyakov and R. S. H. Liu, Photochem. Photobiol. Sci., 2006, 5, 874 RSC.
- J. Saltiel, T. S. R. Krishna, A. M. Turek and R. J. Clark, Chem. Commun., 2006, 1506 RSC.
- J. Saltiel, T. S. R. Krishna and R. J. Clark, J. Phys. Chem. A, 2006, 110, 1694 CrossRef CAS.
- T.-A. V. Khuong, G. Zepeda, C. N. Sanrame, H. Dang, M. D. Bartberger, K. N. Houk and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2004, 126, 14778 CrossRef CAS.
- T. Odani, A. Matsumoto, K. Sada and M. Miyata, Chem. Commun., 2001, 2004 RSC.
- Isomerization of 1,3-dienes in solution: J. H. Pinckard, B. Wille and L. Zechmeister, J. Am. Chem. Soc., 1948, 70, 1938 Search PubMed; J. Saltiel, L. Metts and M. Wrighton, J. Am. Chem. Soc., 1970, 92, 3227 CrossRef CAS; W. A. Yee, S. J. Hug and D. S. Kliger, J. Am. Chem. Soc., 1988, 110, 2164 CrossRef CAS.
- Y. Sonoda, Y. Kawanishi, S. Tsuzuki and M. Goto, J. Org. Chem., 2005, 70, 9755 CrossRef CAS.
- M. D. Cohen, Angew. Chem., Int. Ed. Engl., 1975, 14, 386 CrossRef; A. Gavezzotti, J. Am. Chem. Soc., 1983, 105, 5220 CrossRef CAS; Y. Ohashi, Acc. Chem. Res., 1988, 21, 268 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, crystal structures and CIF files. See DOI: 10.1039/b714792a |
| ‡ CCDC 662879–662881. For crystallographic data in CIF format see DOI: 10.1039/b714792a |
| This journal is © The Royal Society of Chemistry 2008 |
