Titania and silver–titania composite films on glass—potent antimicrobial coatings
Kristopher
Page
a,
Robert G.
Palgrave
a,
Ivan P.
Parkin
*a,
Michael
Wilson
b,
Shelley L. P.
Savin
c and
Alan V.
Chadwick
c
aDepartment of Chemistry, University College London, 20 Gordon Street, London, UK WC1H 0AJ. E-mail: i.p.parkin@ucl.ac.uk
bDivision of Microbial Diseases, UCL Eastman Dental Institute, University College London, 256 Gray's Inn Road, London, UK WC1X 8LD
cSchool of Physical Sciences, Ingram Building, University of Kent, Canterbury, Kent, UK CT2 7NH
First published on 3rd November 2006
Abstract
Titania (anatase) and Ag-doped titania (anatase) coatings were prepared on glass microscope slides by a sol–gel dip-coating method. The resultant coatings were characterised by X-ray diffraction, X-ray absorption near edge structure (XANES), Raman, scanning electron microscopy (SEM), wavelength dispersive X-ray (WDX) analysis, X-ray photoelectron spectroscopy (XPS) and UV-vis techniques and shown to consist of anatase with ca. 0.2–1 atom% Ag2O. Photocatalytic activity of the coatings was determined by photomineralisation of stearic acid, monitored by FT-IR spectroscopy. Photocatalytically-active coatings were screened for their antibacterial efficacy against Staphylococcus aureus (NCTC 6571), Escherichia coli (NCTC 10418) and Bacillus cereus (CH70-2). Ag-doped titania coatings were found to be significantly more photocatalytically and antimicrobially active than a titania coating. No antimicrobial activity was observed in the dark—indicating that silver ion diffusion was not the mechanism for antimicrobial action. The mode of action was explained in terms of a charge separation model. The coatings also demonstrated significantly higher activity against the Gram-positive organisms than against the Gram-negative. The Ag2O–TiO2 coating is a potentially useful coating for hard surfaces in a hospital environment due to its robustness, stability to cleaning and reuse, and its excellent antimicrobial response.
1. Introduction
Staphylococcus aureus is a Gram-positive bacterium which colonises approximately 30% of individuals in developed countries, mainly in the nose or on the skin.1,2 In a colonisation of this type most people experience no symptoms or any infection, however it is able to cause a variety of diseases ranging from the trivial (e.g. boils) to the life-threatening (e.g. toxic shock syndrome). Most S. aureus infections can be treated with antibiotics1 as these are due to infection by methicillin-sensitive S. aureus (MSSA). However, some strains of the organism (known as methicillin-resistant S. aureus—MRSA) are resistant to a number of antibiotics, and infections due to such strains are very difficult to treat.2 MRSA infections are more common in hospital environments where the organism is usually passed on by direct contact, usually by the hands of health care workers (nosocomial infection).2–4S. aureus has achieved methicillin resistance by evolving both an efflux mechanism, which actively and non-specifically expels antibiotics from the cell,5 and by the production of an altered penicillin binding protein PBP2a the product of the mecA gene which is insensitive to methicillin.6 The spread of MRSA and other infections can be controlled effectively through a rigorous hygiene regime. Simple hand-washing is sufficient to help control the spread of the organism,2,7 however this is of little use if the hospital environment is heavily contaminated.3 Contamination of surfaces touched by health care staff in the hospital environment is obviously a potential reservoir for nosocomial infection by MRSA3,4,8,9 and the organism can survive for up to 9 weeks when it dries onto surfaces.4 An antimicrobial coating that actively disinfects hard surfaces touched by nursing staff will help to break the nosocomial infection loop. Such a coating would be particularly useful as a means of disinfection in high traffic communal areas and on items such as door handles, taps and toilet flushes. An effective antimicrobial coating would not necessarily be limited to these areas, but could be employed in various roles across the hospital in both surgical and communal areas.Titanium dioxide (TiO2) is receiving considerable research interest as a photocatalyst and consequently an antimicrobial coating. TiO2 first came to the attention of the scientific community when Fujishima and Honda demonstrated the photolysis of water by a TiO2–Pt electrochemical photocell in 1972.10,11 However it was not until 1985 that the efficacy of TiO2 semiconductor particles as a means of microbial disinfection was first realised by Matsunaga et al.12 It was found that platinised TiO2, when irradiated with ultra band gap UV radiation, acted as an antimicrobial agent, as a result of photocatalytic processes taking place on the TiO2 surface. Mills and LeHunte have written a key review in this area covering photocatalytic and antimicrobial properties of titanium dioxide and metal-doped titanium dioxide thin films.13
Anatase titanium dioxide has a band gap energy (Eg) of 3.2 eV.13 Irradiation of anatase TiO2 with UV radiation greater than Eg causes promotion of an electron from the valence band to the conduction band. This results in the formation of an electron–hole pair. This is a free electron in the conduction band, and a hole in the valence band.13–16 These reactive species then participate in oxidation and reduction processes either within the TiO2 itself (electron and hole recombination), or with adsorbates at the surface. Disinfection of a surface by photocatalysed reactions on TiO2 is a popular possible alternative to using chemical disinfectants such as chlorine bleach.
The effectiveness of the TiO2 as a photocatalyst is in part dependent upon the rate of production of hydroxyl radicals at the surface of the semiconductor. This is in turn dependent upon other factors. These include the energy of the light illuminating the surface and the competition between electron–hole recombination and the redox processes occurring on the surface.17
Titanium dioxide thin films have been formed on glass, steel and other surfaces by a wide range of techniques, especially by sol–gel and chemical vapour deposition.18,19 Furthermore they have been looked at as antimicrobial coatings and shown to be efficient especially under sunlight or black light irradiation.13 Commercial products making use of TiO2 photocatalyst include self cleaning glasses such as Pilkington Activ™ and Saint Gobain Bioclean, self cleaning tiles (TOTO Inc.) and in air purifiers.11 The formation of silver-doped titania thin films has received less attention.20 Silver is incorporated into the titania film by first forming the film, often using a paste method using Degussa P-25, followed by impregnation with an aqueous solution that contains silver ions.21,22 Reduction of this film by photolysis forms nanoparticulate silver nuggets within a host titania matrix. These films have shown to be both more and less active than the parent titania host matrix in the photomineralisation of organic molecules.21,22 The destruction of a particular pollutant has been related to the sensitivity of its radical and the ability of the silver–titania film to stabilise photo-produced electrons and holes. The ability of silver–titania thin films to act as antimicrobial coatings has received scant attention, although one report on preliminary antimicrobial tests showed that the coating halts E. coli colony formation.20 The use of silver as a microbicide is well known and a host of commercial products exist for use in wound dressings, ear-pieces, face masks, catheters, plasters and even for deodorisation of socks.23 A number of commercial antimicrobial surface treatments also exist which rely on the microbicidal activity of the Ag+ ion—these include AgION™ (AgION Technologies Inc.)24 and SilvaGard™ (AcryMed Inc.).25 In all of these instances the silver is impregnated in the products in its nanoparticulate form or as a silver salt such as silver nitrate. The mode of action has been shown to correlate directly with the diffusion of Ag+ into solution. This mode of action works equally well in the dark as in the light as it is not directly related to the photocatalytic mechanism associated with the host titania.
In this paper we report the synthesis of titania and silver-doped titania nanoparticulate thin films from a sol–gel route. We demonstrate that the silver-doped titania thin films are significantly more active than titania films both as a photocatalyst and as an antimicrobial agent when illuminated with 365 nm light. We show that the silver is present in the films as Ag2O by XPS and X-ray absorption spectroscopy (XAS). The silver-doped titania films are rugged and have survived multiple reuses and cleaning with no depletion in antimicrobial effect. We provide a comparison of the antimicrobial efficiencies of the films for Gram-positive, Gram-negative and spore-forming bacteria. Furthermore we observe no antimicrobial activity from these films in the dark, indicating that the mode of action is not, unlike previous studies, due to silver ion diffusion. We conclude that the mode of action of these films is related to the ease of stabilisation of the photo-generated electron–hole pair. These new films are easy to apply at the point of manufacture and have the potential to be used in a clinical environment for reducing bacterial loads and hence nosocomial infections.
Experimental
The chemicals used in this investigation were all purchased from Sigma-Aldrich Chemical Co; propan-2-ol; butan-1-ol; pentane-2,4-dione (acetylacetone); silver nitrate; titanium (IV) n-butoxide and acetonitrile. The thin films were prepared on standard low iron microscope slides (BDH). These were supplied cleaned and polished, but were nonetheless washed with distilled water, dried and rinsed with propan-2-ol and left to air dry before use (2 h).Sol–gel synthesis
General
Characterisation of the synthesised coatings was carried out by field emission scanning electron microscopy (SEM) (Jeol JSM-6301F), wavelength dispersive X-ray (WDX) analysis (Philips ESEM) and by Raman techniques (Renishaw 1000). Powder X-ray diffraction (XRD) was carried out at glancing angles with a 0.5 mm collimator using an AXS D8 Discover instrument equipped with a general area detection diffraction system (GADDS). The TiO2 coating was examined with an angle of incidence of 5° over an angular range of 10–66° for a 15 min period. The Ag–TiO2 coatings were examined with an angle of incidence of 1.5° over an angular range of 10–62.5° for a 30 min period. X-Ray absorption near edge structure (XANES) measurements were made on station 9.3 at the CCLRC Daresbury Synchrotron Radiation Source. The synchrotron has an electron energy of 2 GeV and the average current during the measurements was 150 mA. Ag K-edge extended X-ray absorption fine structure (EXAFS) spectra for the films were collected at room temperature in fluorescence mode using ten films added together to give effectively 20 layers of the sample. Ag2O, AgO, and Ag metal powder were used as standards, along with a Ag metal foil reference, these were collected in standard transmission mode. The standards were prepared by thoroughly mixing the ground material with powdered polyvinylpyrrolidine diluent and pressing into pellets in a 13 mm IR press. Spectra were typically collected to k = 16 Å−1 (k is the wave vector associated with the photoelectron) and several scans were taken to improve the signal-to-noise ratio. For these measurements the amount of sample in the pellet was adjusted to give an absorption of about μd = 1 (where μ is the absorption coefficient and d is the sample thickness). The data were processed in the conventional manner using the Daresbury suite of EXAFS programmes: EXCALIB and EXBACK.26,27 UV-vis spectra were obtained using a Thermo Spectronic Helios Alpha single beam instrument. WDX (Philips ESEM) was performed on carbon-coated samples, and SEM imaging (JEOL JSM-6301F) was performed on gold-coated samples. X-Ray photoelectron spectroscopy (XPS) measurements were carried out on a VG ESALAB 220i XL instrument using focussed (300 µm spot) monochromatic Al-Kα X-ray radiation at a pass energy of 20 eV. Scans were acquired with steps of 50 meV. A flood gun was used to control charging and the binding energies were referenced to surface elemental carbon at 284.6 eV. Depth profile analysis was undertaken using argon sputtering.Photocatalytic activity
The photocatalytic activity of the films was monitored by Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer Paragon 1000). The films were firstly activated by 30 min exposure to UV radiation from a 254 nm germicidal lamp (Vilber Lourmat VL-208G; 8W—BDH/VWR Ltd). The IR spectrum of each stearic acid over-layer was then recorded over the range 3000–2700 cm−1 and the areas of the peaks between 2950–2875 and 2863–2830 cm−1 (the C–H stretching regions of stearic acid) were integrated. Monitoring the integrated area is directly analogous to measuring the concentration of stearic acid on the surface, and so can be used to monitor the degree of photomineralisation after UV irradiation. Slides were irradiated for a set period and then the IR measured after each irradiation. The stearic acid over-layer was applied by dip-coating the sample slides in a 0.02 mol dm−3 solution of stearic acid in methanol. To compare the photocatalytic ability between samples it was ensured that the initial peak areas were as close in value as possible. At the end of the experiments the peak areas were normalised to the initial starting value, such that comparison could be made. Rates of photocatalysis (in molecules cm−2 min−1) were also calculated when the stearic acid decay profile could be fitted to an appropriate rate law.28,29Water droplet contact angle
Photoactive films often demonstrate photoinduced superhydrophilicity (PSH). The degree of PSH can be gauged by observing the change in contact angle of a water droplet on the film surface after UV illumination. The samples were pre-irradiated for 30 min under a 254 nm germicidal lamp (Vilber Lourmat VL-208G—BDH/VWR Ltd), and then a 4 µl droplet of distilled water was placed on the surface. The diameter of the drop was then measured after it had settled. The volume–diameter data were then entered into a computer programme to calculate the contact angle of the water droplet. If a coating demonstrates PSH after UV illumination, the water droplet will be seen to spread out and have a very low contact angle with the coating surface. Droplets were added and measured after every consecutive 30 min of illumination time for 2 h.Antibacterial activity
The antibacterial activity of the films was assessed against Staphylococcus aureus (NCTC 6571), Escherichia coli (NCTC 10418) and Bacillus cereus (CH70-2; mixed vegetative and endospore). Samples were tested in duplicate against a suite of controls (detailed below). Sample coatings and the controls were irradiated under a 254 nm germicidal UV lamp (Vilber Lourmat VL-208G from VWR Ltd; 8 W) for 30 min to both activate and disinfect the films. The sample slides were then transferred to individual moisture chambers (made from Petri dishes with moist filter paper in the base). An overnight culture in nutrient broth (Oxoid Ltd, Basingstoke UK) was then vortexed and 25 µl aliquots of the culture pipetted onto each film in duplicate. The samples were then irradiated by a black-light UV lamp, 365 nm (Vilber Lourmat VL-208BLB; 8W from VWR Ltd) for the desired length of time. The irradiance of the 365 nm lamp was measured at 1.4 mW cm−2 using a Solarmeter Model 5.0 Total UV (A + B) hand held meter (Solartech Inc., Michigan USA). After the desired irradiation period, the bacterial droplets were swabbed from the surface using sterile calcium alginate swabs (Technical Service Consultants Ltd). The swabs were transferred aseptically to 4 ml ‘Calgon’ Ringer solution (Oxoid Ltd, Basingstoke UK) in a glass bijoux containing 5–7 small glass beads. The bijoux was then vortexed until the entire swab had dissolved. For all bijoux, serial 10-fold dilutions of the bacterial suspension were prepared down to 1 × 10−6 in phosphate buffered saline (Oxoid Ltd, Basingstoke UK) in a sterile 96 well plate. Each dilution was then plated in duplicate onto agar. Mannitol salt agar (Oxoid Ltd, Basingstoke UK) was used for S. aureus, MacConkey agar (Oxoid Ltd, Basingstoke UK) was used for E. coli and nutrient agar (Oxoid Ltd, Basingstoke UK) was used for B. cereus. Inoculated plates were then incubated overnight at 37 °C. After incubation a colony count was performed for the dilution with the optimal countable number of colonies (30 to 300 colonies). The data were then processed, taking into account the dilution factor and the mean values of duplicate experiments. The end result is a direct comparison of the number of viable bacteria per ml on the samples to that on a glass control. Experiments were repeated at least twice, giving four data points for each sample tested. Experiments of 2, 4 and 6 h irradiation were conducted for S. aureus; experiments of 6 h were carried out for E. coli and experiments of 2 and 4 h were carried out for B. cereus.Appropriate use of controls is essential in determining whether the coating by itself, UV exposure by itself, or a combination of the two is the cause of any observed microbicidal effect. For each coating under test the following system of positive and negative controls is required: (1) L+S+ (in UV light with an active substrate); (2) L+S− (in UV light with an inactive substrate); (3) L−S+ (in the dark with an active substrate); (4) L−S− (in the dark with an inactive substrate). By using a system of controls as shown it is possible to deduce from the results which conditions result in the antibacterial effect. Photocatalytic coatings should not be antimicrobially active without the activation by UV light, and so only the L+S+ sample should show antibacterial activity. A comparison of L+S+ and L−S− enables kill levels to be calculated. (Note: depending upon the bacterium being investigated, exposure to UV light by itself may have a microbicidal effect. That is to say that the L+S− sample may in some cases demonstrate a measurable kill.)
Results
Synthesis
A simple sol–gel method was used to produce both the TiO2 and silver-doped TiO2 films. The general principle behind this method is the hydrolysis of a titanium precursor and its subsequent polymerisation into a Ti–O–Ti network. By dip-coating the microscope slides, a thin film of titanium precursor is deposited and the gelation of the sol is substantially accelerated.17 Annealing of the samples in a furnace drives off the last traces of solvent, removes carbon and further enhances the polymerisation of the precursor into a crystalline anatase network.The synthetic technique for doping Ag nanoparticles into a titania film was similar to that of the pure titania film. Key to a successful synthesis is the chelation of the metal sites involved; this prevents agglomeration of nanoparticulate Ag and also stops the instant gelation that occurs upon addition of AgNO3 to an acidified titanium precursor. This effect was observed in preliminary experiments without the use of stabilising solvents. Acetylacetone (pentane-2,4-dione) in butan-1-ol was used to stabilise the Ti centre and acetonitrile was used as a coordinating solvent to stabilise the Ag.
Physical characterisation
The TiO2 and Ag-doped TiO2 films had a multicoloured hue, dependent upon the angle from which they are viewed. The appearance of the coatings is due to refringence effects resulting from a small variation in the coating thickness. Films of different thickness were made by varying the number of dip-coats applied—however all films had a uniform appearance, and were smooth. All of the Ag–TiO2 had a bluish–purple hue (possibly due to nanoparticulate silver), with a distinct yellow–orange tinge in certain lighting conditions. Notably TiO2 films without silver did not show the distinct bluish–purple hue or the yellow–orange tinge. Under an optical microscope the surface of the one-coat film TiO2–Ag was featureless, however in the four dip-coat film, cracking of the surface was visible. All thicknesses of the coating were resistant to standard scratch tests with a stainless steel spatula, could not be removed by Scotch® tape and were generally rugged. Indeed the film could only be removed by chipping the glass substrate. Repeated dipping of the coatings into distilled water had no effect on the coating's surface, which could not be wiped off. Depositing coatings onto alternative substrates (brass, aluminium, SnO2, silica and stainless steel) produced films of identical appearance to those made on glass microscope slides. In particular, films deposited onto stainless steel had excellent uniformity and retained the ruggedness and adherence of the films coated on glass. This robust physical behaviour is significantly better than paste,21,22 traditional sol–gel and physical vapour deposition (PVD) prepared titania films and is most akin to those made by chemical vapour deposition (CVD)18,19 such as the commercial products Pilkington Activ and Saint Gobain Bioclean (ca. 25–50 nm thick anatase TiO2, deposited by ‘on-line’ CVD at 650 °C).Characterisation
Powder X-ray diffractograms of the TiO2 films were indexed as anatase (I41/amdz, a = 3.776 Å, c = 9.486 Å). The Ag–TiO2 diffractograms were slightly less well defined than the TiO2 diffractogram but did show peaks attributed to anatase TiO2 (Fig. 1). Furthermore, the Ag–TiO2 patterns exhibited one other significant peak at 31.5° 2θ which was absent in the TiO2 pattern and must therefore be due to the difference in composition—possibly due to the incorporation of a Ag compound rather than crystalline Ag. Database patterns for crystalline Ag do not correlate with this observed peak. The best pattern match for this peak and the remainder of the diffractogram is for the silver oxides AgO and Ag2O. Both silver oxide species correspond well with their most intense peaks aligning with the additional peak observed in the experimental pattern.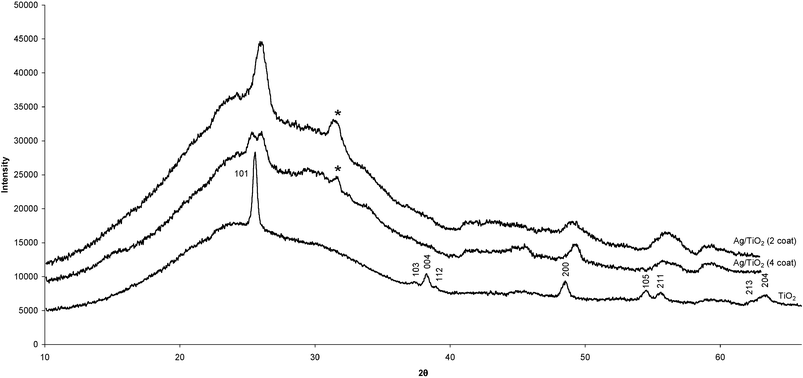 | ||
| Fig. 1 Powder XRD patterns for four coat TiO2 (lower trace) and two and four coat Ag–TiO2 coatings (upper and middle traces respectively). The Ag-oxide peak is marked with an asterisk (*). | ||
Raman analysis of both TiO2 and Ag–TiO2 types was attempted in the range 100 to 1000 cm−1. A characteristic anatase TiO2 scattering pattern was produced (Fig. 2), with a sharp and intense peak at 143 cm−1, and further peaks at 197, 396, 519 and 639 cm−1 in the undoped TiO2 pattern. The less well defined Raman pattern for the Ag-doped samples is most probably due to the lower level of crystallinity in the samples—as observed by the comparatively weak anatase peaks in the XRD. No Raman patterns for silver oxides were apparent. This is most likely due to the low concentration of Ag2O in the films and the poor Raman scattering power of Ag2O compared with the TiO2 matrix.
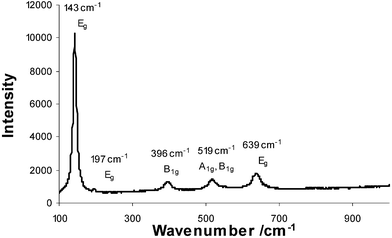 | ||
| Fig. 2 Raman pattern for four coat TiO2 film. | ||
Ag K-edge XAS spectra were collected for the three Ag-doped TiO2 films made from sols with Ag concentrations of 5%, 10% and 20%, Ag metal foil, Ag metal powder, Ag2O and AgO powders. The Ag K-edge XANES data for the doped samples are shown in Fig. 3(a) along with the corresponding data for Ag metal powder, Ag2O and AgO. The energy scales of all the spectra have been consistently normalised to the Ag K-edge at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 eV and the spectra shifted on the y-axis for ease of viewing. Fig. 3(a) shows that the local environment of the Ag atoms has a distinct effect on the shape of the XANES spectra. This can be used to identify the local environment of the Ag atoms in the Ag-doped TiO2 films. In each case, the shape of the XANES spectra for the doped films matches that of the Ag2O standard, indicating that the silver is present in the films as Ag2O [Fig. 3(b)]. The pattern for silver metal—as also shown in Fig. 3(a) is very different to that observed and can't be detected in the samples measured. No bands were observed before the edge in any of the XANES experiments. Furthermore as the XAS gave such a good match to Ag2O [see Fig. 3(b)] it is unlikely that the silver is present within the titania lattice as a discrete solid solution AgxTi2−xO2 because this would give a different edge shape pattern. Hence the films are best described as composites of anatase titania with small amounts of homogeneously distributed silver (I) oxide.
518 eV and the spectra shifted on the y-axis for ease of viewing. Fig. 3(a) shows that the local environment of the Ag atoms has a distinct effect on the shape of the XANES spectra. This can be used to identify the local environment of the Ag atoms in the Ag-doped TiO2 films. In each case, the shape of the XANES spectra for the doped films matches that of the Ag2O standard, indicating that the silver is present in the films as Ag2O [Fig. 3(b)]. The pattern for silver metal—as also shown in Fig. 3(a) is very different to that observed and can't be detected in the samples measured. No bands were observed before the edge in any of the XANES experiments. Furthermore as the XAS gave such a good match to Ag2O [see Fig. 3(b)] it is unlikely that the silver is present within the titania lattice as a discrete solid solution AgxTi2−xO2 because this would give a different edge shape pattern. Hence the films are best described as composites of anatase titania with small amounts of homogeneously distributed silver (I) oxide.
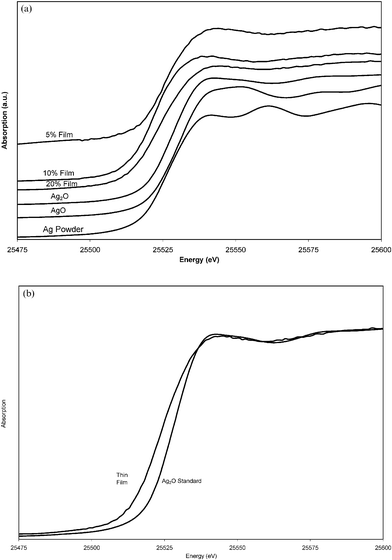 | ||
| Fig. 3 (a) The Ag K-edge XANES for Ag2O, AgO, Ag powder and Ag-doped TiO2 films. No pre edge features were observed; (b) the Ag K-edge XANES for Ag2O and Ag-doped TiO2 films. | ||
SEM and WDX techniques were used to study the composition and morphology of the coated surfaces. WDX analysis confirmed the presence of Ag in the Ag–TiO2 with ratios of 1 part Ag to 100 parts Ti (or less). This was significantly lower than the silver : titania ratio in the starting sol (1 : 10). SEM imaging showed minor shrink cracking in the single or double dip-coated films. The severity of shrink cracking increased with increasing film thickness. At higher magnifications, both coating types had similar morphologies, consisting of granular structures. A high magnification (× 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) image of the two coat Ag-doped coating (Fig. 4) displayed the granular and uneven nature of the coating. In the top left quarter of the image, a high electron density artefact can be observed. This indicates the presence of an agglomerated island that contains Ag since this has a higher electron density than the TiO2 matrix—such islands were seen randomly dispersed across the surface of the film. Also, during the course of the SEM studies, the nanocrystalline nature of the TiO2 coating was observed—particles of 30 nm size on average can be seen in a × 400
000) image of the two coat Ag-doped coating (Fig. 4) displayed the granular and uneven nature of the coating. In the top left quarter of the image, a high electron density artefact can be observed. This indicates the presence of an agglomerated island that contains Ag since this has a higher electron density than the TiO2 matrix—such islands were seen randomly dispersed across the surface of the film. Also, during the course of the SEM studies, the nanocrystalline nature of the TiO2 coating was observed—particles of 30 nm size on average can be seen in a × 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 image (Fig. 5). Observation of particles of this size correlates well with the crystallite sizes calculated by the Scherrer equation from the XRD line broadening—which corresponds best to nanocrystalline titania, rather than a fully crystalline phase. End on SEM studies were also carried out to measure the thickness of the films. The two coat materials had a thickness of approximately 150 nm and a four coat material was approximately twice this thickness, at ca. 300 nm.
000 image (Fig. 5). Observation of particles of this size correlates well with the crystallite sizes calculated by the Scherrer equation from the XRD line broadening—which corresponds best to nanocrystalline titania, rather than a fully crystalline phase. End on SEM studies were also carried out to measure the thickness of the films. The two coat materials had a thickness of approximately 150 nm and a four coat material was approximately twice this thickness, at ca. 300 nm.
 | ||
Fig. 4 SEM image of two coat Ag–TiO2 coating × 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, scale bar 100 nm. 000, scale bar 100 nm. | ||
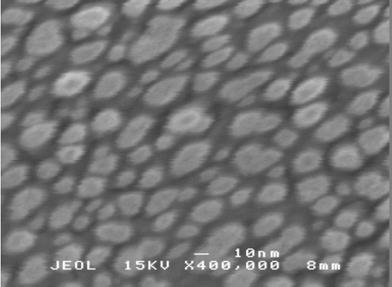 | ||
Fig. 5 SEM image of TiO2 coating × 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, scale bar 10 nm. 000, scale bar 10 nm. | ||
X-Ray photoelectron spectroscopy was undertaken on two sets of four coat Ag–TiO2 films, one on a set exposed to UV light and one on the films as made. Both gave the same XPS profile. The titanium to oxygen atomic ratio was as expected 2 : 1, no other elements were detected other than carbon and silicon at a few atom%. The percentage of the carbon decreased dramatically on etching indicating that it was residual carbon from within the XPS chamber. The Si abundance was constant with etching and probably a result of breakthrough to the underlying glass on regions where there was a small crack in the titania coating, notably it was only seen in one of the four samples analysed. Silver was detected both at the surface and throughout the film and its abundance was invariant with sputter depth. The silver was typically detected at below 1 atom%—significantly lower than that in the initial sol but comparable to that observed by WDX analysis (values ranged around 0.2 atom%, however accurate quantification was difficult at such low levels). The detection limit of the instrument is approximately 0.1 atom% and for quantification it is 0.2 atom%. XPS spectra were collected and referenced to elemental standards. The Ti 2p3/2 and O 1s binding energy shifts of 458.6 eV and 530.1 eV match exactly literature values for TiO2.30 In the sample exposed to UV light just prior to measurement there was a small shoulder to both the Ti and O peaks that correspond to Ti2O3. Interestingly the silver 3d5/2 XPS showed a single environment centred at 367.8 eV which gave a best match for Ag2O (literature reports at 367.7–367.9 eV) rather than for silver metal 368.3 eV (Fig. 6).30 Hence the XPS is consistent with the silver being oxidised as Ag(I) rather than a metallic form in the thin films. Furthermore sputtering studies showed no change in the silver environment with sputter depth. This indicates that the silver is present as Ag2O and not a Ag2O coated Ag particle; as otherwise an asymmetry to the peak shape would have occurred.
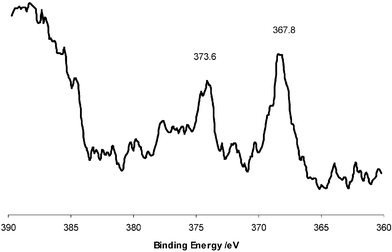 | ||
| Fig. 6 XPS Ag 3d profile for a four coat Ag–TiO2 coating. | ||
UV-vis spectroscopy of the TiO2 and Ag–TiO2 thin films on glass was carried out in the range 300–800 nm. A band edge for the O2− to Ti4+ transition in anatase TiO217 was observed in all of the types of coating at approximately 380 nm. This coupled with XRD and Raman evidence showed that the anatase form of TiO2 was present in all films. An approximate value of the optical band gap for the coatings was obtained by extrapolation on a plot of (ahν)1/2versus hν, where a is the absorbance of the film (a = −log T/T0; T, sample optical transmission; T0, substrate optical transmission) and hν the photon energy. The band gap for the TiO2 coating was in the region of 3.0 eV—which is to be expected for the anatase form of TiO2 (3.2 eV).13 Band gap plots for the Ag-doped coatings were not as easy to interpret as that of TiO2, giving a band gap range of 2.8–3.4 eV. Ag metal nanoparticles could not be detected by the observation of a plasmon band31,32 in the UV visible spectra of the Ag–TiO2 films. However, nanoparticulate silver was detected in the initial starting sol by this method, exhibiting a broad plasmon band at 430 nm. There was, therefore, considering the UV, XAS and XPS spectra, little evidence for the incorporation of these Ag metal nanoparticles into the coatings intact without transformation into an oxide.
Functional properties I: photocatalysis and water droplet contact angle
All of the films showed photocatalytic activity with 254 nm germicidal lamp illumination over a period of eight hours. The reason for choosing the 254 nm (4.88 eV) lamp was to make sure that the radiation was of greater energy than the TiO2 band gap (3.2 eV). The degree of photocatalysis observed varied between the different coatings, as shown in Fig. 7. It can be clearly seen that the Ag-doped coatings were significantly more photocatalytically active than the undoped TiO2 coating of the same thickness. Amongst the different thickness Ag-doped coatings there was also a difference in the photocatalytic activity. The two-coat Ag–TiO2 film had the highest initial rate of photocatalysis. The zero order rate constants for the degradation of stearic acid were calculated at 4.05 × 1012 molecules cm−2 min−1 for TiO2 and 5.85 × 1012 molecules cm−2 min−1 for a Ag2O–TiO2 coating of the same thickness. The photoactivity of the TiO2 films generated in this study to photomineralise stearic acid was slightly lower than our previous work using CVD and sol–gel prepared films.17 In previous work depositions had been conducted on barrier glass which has a diffusion layer to stop sodium ion diffusion from the glass substrate into the film. It has been noted previously that sodium diffusion during calcinations can reduce the photocatalytic ability of titania films.28,33 However our XPS and WDX studies did not detect any sodium in the titania films so if present it must be less than the 0.1 atom% detection limit of these techniques.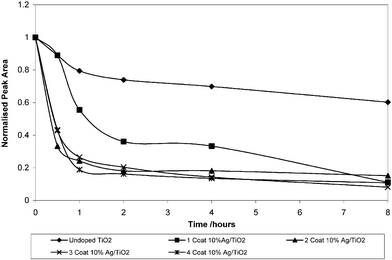 | ||
| Fig. 7 Relative photocatalytic abilities of all coatings. | ||
Initial water contact angle measurements showed that all of the samples were hydrophilic as they made ca. 15° water contact angles and they became superhydrophilic upon exposure to UV radiation. The Ag–TiO2 samples had contact angles of around 1° after only the initial 30 min of irradiation with 254 nm. These angles decreased further upon subsequent exposure to the germicidal lamp (254 nm)—but as they were so low they were difficult to quantify. However, it showed that the 2-coat Ag–TiO2 film had a very high degree of photoinduced superhydrophilicity, as did the three and four coat versions of the same coating. Photoinduced superhydrophilicity was not observed in the coatings deposited onto metal substrate materials, with initial contact angles being significantly higher (ca. 20°) than for equivalent coatings on glass. This may be due to metal ions diffusing into the coating during the annealing step.
Functional properties II: microbicidal activity
The antimicrobial activity of the coatings was assessed against three different micro-organisms; Staphylococcus aureus (NCTC 6571), Escherichia coli (NCTC 10418) and Bacillus cereus (CH70-2). These organisms represent a spectrum of different classes of bacterium. S. aureus is perhaps the most important target for this investigation, because of its direct link with MRSA and hospital acquired infections. S. aureus is also a fairly typical example of a Gram-positive organism, so it serves as a useful indicator of the behaviour of a sample coating towards this class of micro-organism. In the interests of completeness and experimental rigour, the coatings were also tested against E. coli, a Gram-negative organism and with B. cereus, a Gram-positive spore-forming organism. It should be noted that the same coatings were reused for all antimicrobial testing and that all experiments were carried out in duplicate and repeated twice. The samples were cleaned between uses by wiping with isopropanol wipes (as commonly used to clean hard surfaces in hospitals). The Ag-doped coatings performed very well under conditions of reuse, maintaining a constant level of effectiveness despite being handled, cleaned and reused.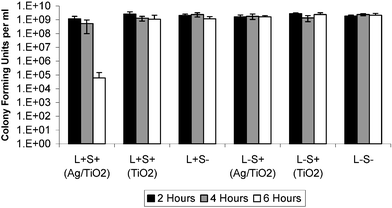 | ||
| Fig. 8 Bacterial kills for the two coat Ag–TiO2 sol–gel prepared coating against Staphylococcus aureus after 2, 4 and 6 h illumination times with 365 nm radiation. The viable counts are expressed as colony-forming units ml−1. L+S+ refers to the exposure of an active coating (identity in brackets) to UV light. L+S− refers to the exposure of an uncoated slide to UV light. L−S+ refers to an active coating (identity in brackets) kept in the dark and L−S− refers to an uncoated slide kept in the dark. | ||
Discussion
There are a number of avenues that can be followed in an attempt to provide an explanation for the enhanced activity of the Ag-doped TiO2 coating over that of an un-doped TiO2 film. In reality, there is likely no one single reason for the increased activity, rather the observation results from a combination of effects. The simplest explanation is one of surface microstructure. The Ag-doped films displayed islands with a high silver density. This in itself is a good explanation for the difference in activities, but it does not take into account other evidence from the characterisation of the coatings. XRD, XPS and XANES analysis elucidated the presence of the silver oxide Ag2O. It is possible that these species act as a source of electrons and as charge separators because of their high electron density relative to the TiO2 matrix. These factors would enhance the overall photoactivity of the coating by firstly donating extra electrons to the conduction band which in turn are able to produce more reactive species at the catalyst surface, and secondly by blocking electron–hole recombination which stops the production of radicals at the surface. Indeed, this explanation is supported by the photocatalysis results. There have also been reports in the literature of some silver oxides exhibiting semiconductor behaviour35,36 and Ag2O is quoted in the literature as having a band gap of 2.25 eV (550 nm).37 This may go some way to explaining the apparent change in the optical band gap of the Ag–TiO2 films over the TiO2 coating.It is difficult to compare photocatalysis results with the literature since there is not as yet an agreed universal reference against which photocatalysis can be measured. However, the use of Pilkington Activ™ glass (which is TiO2 coated) as a reference photocatalyst has been proposed, since this would make a reliable standard.38 Preliminary photocatalytic results in our laboratory indicate the Ag–TiO2 films are considerably more active. It is equally difficult to compare the microbiological results of this investigation with other work in the literature because of the great diversity in techniques used, and in the precise details of the experiments performed. The vast majority of studies of TiO2 antimicrobials are carried out in solution using a suspension of Degussa P25 TiO2.14,15,38 This is fundamentally different from the thin film coatings prepared in this study because the surface area of active catalyst in suspension would be significantly greater than that available on a thin film surface (perhaps up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times greater). Furthermore, titania particles in suspension can be ingested by cells via phagocytosis—this has been shown to cause rapid cellular damage in addition to that caused by photocatalysis.39,40 Consequently, literature results from this method differ greatly from those obtained in this study. Most studies also examined only E. coli. However, the efficacy against E. coli when using a suspended powder is variable from study to study. One study used an inoculum of 1 × 106 cfu ml−1E. coli in a P25 suspension and observed 85% effectiveness after 20 min exposure to UV (peak wavelength 356 nm), and 100% effectiveness after an hour.39 This compares with 69% effectiveness against 1 × 109 cfu ml−1E. coli after 6 h UV illumination (365 nm) for the two-coat Ag–TiO2 coating prepared in this study.
000 times greater). Furthermore, titania particles in suspension can be ingested by cells via phagocytosis—this has been shown to cause rapid cellular damage in addition to that caused by photocatalysis.39,40 Consequently, literature results from this method differ greatly from those obtained in this study. Most studies also examined only E. coli. However, the efficacy against E. coli when using a suspended powder is variable from study to study. One study used an inoculum of 1 × 106 cfu ml−1E. coli in a P25 suspension and observed 85% effectiveness after 20 min exposure to UV (peak wavelength 356 nm), and 100% effectiveness after an hour.39 This compares with 69% effectiveness against 1 × 109 cfu ml−1E. coli after 6 h UV illumination (365 nm) for the two-coat Ag–TiO2 coating prepared in this study.
The antimicrobial effect of titania coatings is derived from the production of hydroxyl radicals,15,16 hence a rationalisation for the relative effectiveness of the coating against Gram-positive and Gram-negative organisms can be offered. Previous research examining the toxicity mechanism of TiO2 against micro-organisms showed that the lethal action involved breach of the cytoplasmic membrane and the resultant leakage of intracellular components.39,41 For this to occur, the hydroxyl radicals produced at the coating surface must be able to directly attack the cytoplasmic membrane. The differing morphologies of Gram-positive and Gram-negative cell envelopes means that the passage of hydroxyl radicals from coating surface to cytoplasmic membrane is hindered to differing extents. For S. aureus, the only barrier is the peptidoglycan layer and the periplasmic space. Despite having a thick layer of peptidoglycan, S. aureus is likely afforded little protection from the hydroxyl radicals. This is because the peptidoglycan is composed of a fairly open network polymer of N-acetylmuramic acid and N-acetylglucosamine polysaccharide chains, with peptide bridges. In contrast, the passage of hydroxyl radicals towards the cytoplasmic membrane of E. coli is significantly hindered by the morphology of the cell envelope. In Gram-negative organisms, such as E. coli, the cytoplasmic membrane is protected by a thin layer of peptidoglycan, followed by an outer membrane. The outer membrane presents a significant barrier to hydroxyl radical passage since it is comprised of a complex layer of lipids, lipopolysaccharides and proteins. The outer membrane layer presents an attractive target for approaching hydroxyl radicals because of this composition. Although the outer membrane is semi-permeable, many of the hydroxyl radicals will react with the lipid constituents of the membrane rather than pass through it. Once the membrane is breached, however, there are no further significant obstacles blocking the approach of the radicals to disrupt the cytoplasmic membrane and cell death can be observed.39 This interpretation is supported by a recent study of the photokilling of E. coli by TiO2 thin films.40 The bactericidal action was found to be a two step process in which the outer membrane is compromised first, followed by the cytoplasmic membrane. Hence the Gram-negative envelope affords better protection against the hydroxyl radical as a cytotoxic agent. This rationale would therefore account for the higher antimicrobial activity of the Ag–TiO2 towards Gram-positive organisms than Gram-negative.
In the films prepared here, no antibacterial activity was observed from the Ag–TiO2 films in the absence of light—this implies that the silver has no direct role in promoting increased bacterial kills. The presence of silver as an oxide within the film enhanced the antimicrobial and photocatalytic properties. Solutions of silver sols have applications as antibacterial agents where the active component of these solutions is the Ag+ ions which disrupt bacterial metabolism.42 The silver sols display a large surface area and are known to be partially oxidised by atmospheric oxygen to give Ag2O. While this is only sparingly soluble in water it is sufficient to provide antibacterial effects. These antibacterial effects are manifested independently of whether a light source is used or not. In the films made in this study no bacterial kill was observed in the dark. This is strong evidence that the films are not functioning as microbicides due to the presence of silver ions, as we would have observed some kill in the absence of light. Silver metal is normally quite resistant to oxidation in air and requires stronger oxidising agents such as ozone to convert to the oxide. The fact that the silver is present as Ag2O in these films is a consequence of the high temperature anneal and the fact that the silver is embedded in a titanium dioxide matrix. Although the silver is present as the oxide, UV illumination of titania can in principle convert this to the native metal in the presence of titanium dioxide. However XPS studies of the Ag2O–TiO2 film both before and after UV irradiation did not show any change in the silver environment—the binding energy shifts match well for Ag2O and no lower energy peak was seen as would be characteristic of silver formation. Hence this combined with the lack of any antibacterial activity in the dark seems, even after 12 cycles of UV irradiation, to indicate that any possible formation of silver metal in this system occurs below the detection limits of the experiments used. Recent work has shown that at elevated temperature in the presence of oxygen the most stable thermodynamic form is Ag2O.43 This correlates nicely with what was observed in this study. However, the presence of the silver oxide Ag2O in conjunction with titania did show a marked enhancement over a pure titania film as a photocatalyst. This is most likely due to stabilisation of photogenerated electron–hole pairs at the titania surface by localisation of the photogenerated electron onto the silver oxide.
Conclusion
Photocatalytically-active and antimicrobially-active coatings were synthesised by a simple sol–gel dip-coating technique. The resultant coatings were characterised by glancing angle X-ray diffraction, XPS, XANES, Raman spectroscopy, SEM, WDX and UV-vis spectroscopy and shown to consist of anatase titania with embedded Ag2O particles. Photocatalytic activity of the coatings was determined by photomineralisation of stearic acid and monitored by FT-IR spectroscopy. Coatings demonstrating high photocatalytic activity against stearic acid were then screened for antibacterial efficacy against Staphylococcus aureus (NCTC 6571), Escherichia coli (NCTC 10418) and Bacillus cereus (CH70-2). Ag-doped coatings were found to be significantly more photocatalytically and antimicrobially active than a regular TiO2 coating. This was explained in terms of a charge separation model. Notably the coatings showed no activity against bacteria in the dark—indicating that their efficacy is not due to silver ions acting as a microbicide. The coatings also demonstrated significantly higher activity against the Gram-positive organisms than against the Gram-negative. This was explained in terms of the comparative morphologies of the cell envelopes and the permeability of these envelopes to the likely toxic agent, the hydroxyl radical. The two coat Ag–TiO2 coating would appear to be a potentially useful coating for hard surfaces in a hospital environment due to its robustness, stability to cleaning and reuse, and its excellent antimicrobial response to all organisms tested thus far. Such a coating would need to be applied at the point of manufacture of a particular item—and could not be retrofitted to existing surfaces because of the heat treatment required to generate the active coatings. However on new products it could create a very potent antimicrobial coating.Acknowledgements
The Horshall fund is thanked for financial support. Professor Parkin is a Royal Society Wolfson Trust merit holder. K.P. would like to thank Ms Valérie Decraene for her help and advice during the antimicrobial testing. Mr Kevin Reeves is thanked for his assistance with SEM imaging and WDX analysis. CCLRC Daresbury is thanked for provision of XANES time.References
- Health Protection Agency UK Staphylococcus aureushttp://www.hpa.org.uk/infections/topics_az/staphylo/menu.htm, last accessed 10th January 2006 Search PubMed
.
- NHS Direct NHS Direct Online Health Encyclopaedia: MRSA http://www.nhsdirect.nhs.uk/articles/article.aspx?articleId=252, last accessed 10th January 2006
.
- S. J. Dancer, J. Hosp. Infect., 2004, 56, 10 CrossRef CAS
.
- B. Hota, Clin. Infect. Dis., 2004, 39, 1182 CrossRef
.
-
L. M. Prescott, J. P. Harley and D. A. Klein, Microbiology, Mc Graw-Hill, Boston, 6 edn, , 2002 Search PubMed
.
- H. de Lencastre, B. L. M. de Jonge, P. R. Matthews and A. Tomasz, J. Antimicrob. Chemother., 1994, 33, 7 CAS
.
- S. Oie, I. Hosokawa and A. Kamiya, J. Hosp. Infect., 2002, 51, 140 CrossRef CAS
.
- J. M. Boyce, G. Potter-Bynoe, C. Chenevert and T. King, Infect. Cont. Hosp. Epidemiol., 1997, 18, 622 Search PubMed
.
- D. Talon, J. Hosp. Infect., 1999, 43, 13 CrossRef CAS
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CAS
.
- A. Fujishima and X. Zhang, C. R. Chim., 2006, 9, 750 CrossRef CAS
.
- T. Matsunaga, R. Tomoda, T. Nakajima and H. Wake, FEMS Microbiol. Lett., 1985, 29, 211 CrossRef CAS
.
- A. Mills and S. LeHunte, J. Photochem. Photobiol., A, 1997, 108, 1 CrossRef CAS
.
- T. Matsunaga, R. Tomoda, T. Nakajima, N. Nakamura and T. Komine, Appl. Environ. Microbiol., 1988, 54, 1330 CAS
.
- J. C. Ireland, P. Klostermann, E. W. Rice and R. M. Clark, Appl. Environ. Microbiol., 1993, 59, 1668 CAS
.
- W. A. Jacoby, P. C. Maness, E. J. Wolfrum, D. M. Blake and J. A. Fennell, Environ. Sci. Technol., 1998, 32, 2650 CrossRef CAS
.
- A. Rampaul, I. P. Parkin, S. A. O'Neill, J. DeSouza, A. Mills and N. Elliott, Polyhedron, 2003, 22, 35 CrossRef CAS
.
- H. M. Yates, M. G. Nolan, D. W. Sheel and M. E. Pemble, J. Photochem. Photobiol., A, 2006, 179, 213 CrossRef CAS
.
- A. C. Jones, T. J. Leedham, P. J. Wright, M. J. Crosbie, K. A. Fleeting, D. J. Otway, P. O'Brien and M. E. Pemble, J. Mater. Chem., 1998, 8, 1773 RSC
.
- M. Machida, K. Norimoto and T. Kimura, J. Am. Ceram. Soc., 2005, 88, 95 CAS
.
- B. F. Xin, L. Q. Jing, Z. Y. Ren, J. Q. Wang, H. T. Yu and H. G. Fu, Acta Chim. Sin., 2004, 26, 1110 Search PubMed
.
- X. F. You, F. Chen, J. L. Zhang, J. S. Huang and L. Z. Zhang, Cuihua Xuebao, 2006, 27, 270 CAS
.
- C. Radheshkumar and H. Münstedt, React. Funct. Polym., 2006, 66, 780 CrossRef CAS
.
- AgION Technologies Inc. Agion™ Technology http://www.agion-tech.com, last accessed 10th January 2006
.
- AcryMed Inc. SilvaGard™
http://www.acrymed.com/techATD.htm, last accessed 5th June 2006
.
-
N. Binsted, J. W. Campbell, S. J. Gurman and P. C. Stephenson, SERC Daresbury Program Library, 1992, CCLRC Daresbury Laboratory, Warrington, UK Search PubMed
.
-
N. Binsted, EXCURV98: CCLRC Daresbury Laboratory computer program, 1998, CCLRC Daresbury Laboratory, Warrington, UK Search PubMed
.
- Y. Paz, Z. Luo, L. Rabenberg and A. Heller, J. Mater. Res., 1995, 10, 2842 CrossRef CAS
.
- G. Hyett, M. Green and I. P. Parkin, J. Am. Chem. Soc., 2006, 128, 12147 CrossRef CAS
.
- NIST X-ray photolectron spectroscopy database http://srdata.nist.gov/xps/, last accessed 10th January 2006
.
- N. L. Pickett and P. O'Brien, Chem. Rec., 2001, 1, 467 CrossRef CAS
.
- W. L. Barnes, A. Dereux and T. W. Ebbesen, Nature, 2003, 424, 824 CrossRef CAS
.
- Y. Paz and A. Heller, J. Mater. Res., 1997, 12, 2759 CrossRef CAS
.
- W. A. Rutala, E. B. S. Katz, R. J. Sherertz and F. A. Sarubbi, J. Clin. Microbiol., 1983, 18, 683 CAS
.
- J. A. McMillan, Chem. Rev., 1962, 62, 65 CrossRef CAS
.
- R. Haul, Z. Phys. Chem., 1994, 186, 227 CAS
.
- L. A. Peyser, A. E. Vinson, A. P. Bartko and R. M. Dickson, Science, 2001, 291, 103 CrossRef CAS
.
- A. Mills, A. Lepre, N. Elliott, S. Bhopal, I. P. Parkin and S. A. O'Neill, J. Photochem. Photobiol., A, 2003, 160, 213 CrossRef CAS
.
- Z. Huang, P. C. Maness, D. M. Blake, E. J. Wolfrum, S. L. Smolinski and W. A. Jacoby, J. Photochem. Photobiol., A, 2000, 130, 163 CrossRef CAS
.
- K. Sunada, T. Watanabe and K. Hashimoto, J. Photochem. Photobiol., A, 2003, 156, 227 CrossRef CAS
.
- Z. X. Lu, L. Zhou, Z. L. Zhang, W. L. Shi, Z. X. Xie, H. Y. Xie, D. W. Pang and P. Shen, Langmuir, 2003, 19, 8765 CrossRef CAS
.
- A. B. G. Lansdown, Bull. R. Coll. Pathol., 2006, 133, 36 Search PubMed
.
- W. X. Li, C. Stampfl and M. Scheffler, Phys. Rev. B, 2003, 68, 165412 CrossRef
.
| This journal is © The Royal Society of Chemistry 2007 |
