Sunscreens with an absorption maximum of ≥360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts
Gabriele
Vielhaber
a,
Susanne
Grether-Beck
c,
Oskar
Koch
a,
William
Johncock
b and
Jean
Krutmann
*c
aSymrise GmbH & Co. KG, Mühlenfeldstr. 1, D-37603, Holzminden, Germany
bSymrise GmbH & Co. KG, Bleichenbruecke 10, D-20354, Hamburg, Germany
cInstitut für umweltmedizinische Forschung, Auf'm Hennekamp 50, D-40225, Düsseldorf, Germany. E-mail: krutmann@.uni-duesseldorf.de; Fax: ++49-211-3389-226; Tel: ++49-211-3389-224
First published on 7th February 2006
Abstract
UVA1-induced expression of matrix metalloproteinase-1 (MMP-1) is mediated by an autocrine mechanism involving the cytokines interleukin-1 and -6 (IL-1 and IL-6). The subsequent degradation of collagen fibers is thought to be the main cause of skin wrinkling. As it is currently not known which wavelengths within the UVA1 range are responsible for these effects, we have assessed 5 UVA1 filters (experimental filters HRH21328 and HRH22127, butyl methoxydibenzoylmethane (BMDM), diethylaminohydroxybenzylbenzoic acid hexyl ester (DHBB) and anisotriazine) with different absorption maxima for their capacity to protect against UVA1-induced MMP-1 expression. To test the efficacy of these hydrophobic filters in a cell culture system, UVA1 irradiation of primary human fibroblasts was performed through a quartz microplate filled with ethanolic solutions of the UVA filters placed on top of the cell microplate. Inhibition of UVA1-induced gene expression was detected by real time RT-PCR. The efficacy to protect against UVA1-induced MMP-1 expression was wavelength dependent: the protection by HRH22127 was best, followed by HRH21328, DHBB, BMDM, and anisotriazine. In addition, HRH22127 and HRH 21328 both significantly inhibited UVA1-induced expression of IL-1α and IL-6 with HRH21238 being superior to HRH22127. These studies indicate that UVA1 filters with a maximum absorption at ≥360 nm are most effective in preventing UVA1 radiation-induced MMP-1, IL-1α, and IL-6 expression pointing towards a critical role for effective filtering beyond ≥360 nm for protection against UVA1-induced photoaging.
 Gabriele Vielhaber Gabriele Vielhaber | Gabriele Vielhaber received her Diploma degree in chemistry at the University of Heidelberg in 1992 and her Dr. rer. nat. for biochemical and cell biological work on spingolipid metabolism from the University of Bonn in 1996. She started her professional career at Beiersdorf where she worked on immunomicroscopical methods for localization of skin lipids and proteins. In 2000 she joined Haarmann & Reimer, now Symrise, where she is now responsible for the research and development of cosmetic ingredients with special emphasis on agents for skin barrier repair, anti-irritants, modulators of melanogenesis and photoprotectives. |
 Susanne Grether-Beck Susanne Grether-Beck | Susanne Grether-Beck received her Diploma degree in biology from the University of Freiburg, Germany, in 1987 for studies of the physiology of phototrophic bacteria in the laboratory of Prof. Jürgen Oelze. In 1993 she obtained her Dr. rer. nat. degree for biochemical and molecular biological studies on the nicotine regulon of Arthrobacter nicotinovorans with Prof. Karl Decker at the Biochemical Institute at Freiburg University. She joined the group of Dr Jean Krutmann as a research fellow. Currently with the Institut für Umweltmedizinische Forschung (IUF) at the Heinrich-Heine-University Düsseldorf her research focuses on the UV-induced signalling in human skin cells and the development of protective strategies against UV-induced skin damage. |
 Oskar Koch Oskar Koch | Oskar Koch studied chemistry at University of Göttingen and received his Diploma degree in 1981. He moved to the University of Hannover where he received his Dr. rer. nat. for a thesis on cycloaddition reactions of fulvene derivatives in 1985. In 1986 he joined Haarmann & Reimer GmbH, now Symrise. During two decades in R & D his main field of research has been the development of organic UV filters and the optimization of their manufacturing processes. Based on this experience he is now responsible for the synthesis and process development of new cosmetic ingredients at Symrise. |
 William Johncock William Johncock | William Johncock obtained his PhD in organic chemistry from the University of Wales in 1981 in which he discovered novel photochromic systems. This was followed by a post doctoral work at the University of Geneva working on reactive carbene intermediates. He then joined Procter & Gamble where he developed consumer products. In 1989 he joined Givaudan where he began his career in marketing of specialty raw materials for the cosmetic industry focussing on sun protection. In 1996 he joined Haarmann & Reimer, now Symrise, where he was initially responsible for marketing of cosmetic ingredients. He is now in charge of the business units UV Protection and Menthols for Symrise. |
 Jean Krutmann Jean Krutmann | Jean Krutmann is Professor of Dermatology and Environmental Medicine and Director of the Institute für Umweltmedizinische Forschung (IUF) at the Heinrich-Heine-University Düsseldorf. His research is in the field of dermatotoxicology, immunodermatology and molecular aging with special emphasis on environmentally-induced skin diseases. He is author or co-author of more than 200 papers. He is recipient of the International Arnold-Rikli-Award, the Albert Fleckenstein Award, The Paul Gerson Unna Award, The Oscar Gans Award, the C.E.R.I.E.S. Research Support Award and the Dermopharmacy Innovation Award. He currently also serves as Vice-Dean for Research and as Director of the DFG-Postgraduate-School of “Molecular Mechanisms in Ageing and Targets for Prevention of Ageing”. |
Introduction
Ultraviolet (UV) exposure of human skin induces multiple deleterious effects in the epidermis and dermis. UVB (290–320 nm) radiation primarily causes photocarcinogenesis due to its direct interaction with cellular DNA and subsequent formation of cyclobutane pyrimidine dimers, 6–4 photoproducts and thymine glycols.1 The major consequence of UVA (320–400 nm) radiation is the generation of reactive oxygen species, which, however, can also induce cancer by e.g. generation of oxidized DNA base derivatives like 8-hydroxydeoxyguanosine.2Whereas UVB effects are mainly restricted to the epidermis, UVA rays directly affect the dermal compartment and are therefore thought to be the major factor responsible for photoaging of human skin. It had been shown that the long wave part of UVA, UVA1 (340–400 nm) especially accounts for damaging effects in human dermal fibroblasts, as induction of cytokines,3 matrix metalloproteinases,4,5 and mtDNA mutations.6 Of these, the induction of collagenase (matrix metalloproteinase-1, MMP-1) which degrades collagen type I, the major constituent of the connective tissue, is of particular significance since the extent of collagen I reduction correlates with photodamage in human skin.7 Wlaschek et al. demonstrated that the UVA1-induced up-regulation of MMP-1 is regulated by interrelated autocrine loops of interleukin-1 (IL-1) and interleukin-6 (IL-6):3,8 UVA1 radiation rapidly induces bioactivity of extracellular IL-1α and IL-1β (two-fold increase of IL-1 at 3 h) which then induce IL-6 expression. IL-1 and IL-6 both enhance the biosynthesis of MMP-1, peaking at 24 h.3 Concomitantly, the de novo synthesis of IL-1 is stimulated leading to a further boost of IL-6 and MMP-1 induction.
The UVA1 lamps used in these studies emit in the 340–440 nm range. It is not currently known whether the whole UVA1 range is of equal importance for all biological consequences or whether there are selected wavelength ranges that especially account for selected biological effects. This information, however, is critical for the design of strategies to protect human skin from solar UV radiation such as UV filters. Since UVA sources emitting single wavebands in the UVA1 range >360 nm are not readily available, we aimed to use UVA1 filters with different absorption profiles to approximate selected wavelength ranges.
To date there are only two organic UVA1 absorbers commercially available: the broad band filter anisotriazine which absorbs between 270–380 nm (E1%/1cm = 790) and butyl methoxydibenzoylmethane (BMDM) which absorbs between 310–390 nm (abs. max. = 357 nm, E1%/1cm = 1180) (Table 1). In addition, physical sunscreens based on titanium dioxide and/or zinc oxide are efficient UVA1 filters.9 The protective effect of these UVA1 absorbers in vivo has been demonstrated: BMDM was shown to prevent UV-induced immunosuppression and UVA-induced p53 expression.10,11 Augustin et al. found that isopropyl dibenzoylmethane (abs. max. = 350 nm) did not prevent UVA-induced IL-1α release but together with BMDM and an UVA2 absorber it provided protection in a three-dimensional skin equivalent against cytotoxicity and IL-1α release.12 In addition, a titanium oxide–titanium dioxide preparation was highly protective. In another study, Diffey and coworkers investigated 59 different sunscreen formulations on their protective capacity based on SPF, protection against erythema, photostability and the critical wavelength value, which was defined as the wavelength below which 90% of the area of the spectral absorption spectrum from 290–400 nm was found.9 They found a linear relationship between the critical wavelength and the erythema protection for products with similar SPF values. Only 10% of the products had a critical wavelength of 370 nm or more and only in combination with a SPF > 8 was a sufficient erythema protection achieved. Thus, wavelengths >370 nm seem to be of critical importance for cutaneous photodamage.
Yet, sunscreens significantly absorbing in the far UVA range (370–400 nm) are so far unavailable . We therefore developed two new sunscreens, HRH22127 (absorption range 290–400 nm, abs. max. = 361 nm, E1%/1cm = 770) and HRH21328 (absorption range 285–410 nm, abs. max. = 373 nm, E1%/1cm = 730) (Table 1). Both sunscreens are oil-soluble and exhibit excellent photostability.
To test the efficacy of these hydrophobic filters in a cell culture system, we have developed a new experimental design using a quartz microplate of exactly the same dimensions as the cell microplate. The quartz microplate was placed on top of the cell microplate and filled with ethanolic solutions of the sunscreens. This allowed us to measure the UVA1-induced expression of MMP-1, IL-1 and IL-6 in cultured human dermal fibroblasts in the presence of HRH22127 or HRH21238 in comparison to BMDM, anisotriazine and diethylaminohydroxybenzylbenzoic acid hexyl ester (DHBB) (absorption range 310–390 nm, abs. max. = 354 nm, E1%/1cm = 930) (Table 1).
| Filter No. | Name | Absorption spectrum | Abs. max. (nm)/UV absorption (E1%/1cm) |
|---|---|---|---|
| 1 | Anisotriazine |

|
310–340/790 |
| 2 | Diethylaminohydroxybenzylbenzoic acid hexyl ester (DHBB) |

|
354/930 |
| 3 | Butyl methoxydibenzoylmethane (BMDM) |

|
357/1180 |
| 4 | HRH22127 |

|
361/770 |
| 5 | HRH21328 |

|
373/730 |
Materials and methods
Materials
The quartz microplate was obtained from Hellma, Müllheim, Germany. HRH22127, HRH21328, DHBB and BMDM were from Symrise, Holzminden, Germany. Anisotriazine was from Ciba Specialty Chemicals, Grenzach, Switzerland. All chemicals were of analytical grade (>99% purity). The filters were added to the quartz plate using a 500–5000 µL variable pipette (Research, Eppendorf AG, Hamburg, Germany).Cell culture
Primary human dermal fibroblasts were cultured in Earle's Minimum Essential Medium (EMEM) without glutamine (PAA Laboratories GmbH, Cölbe, Germany), supplemented with antibiotics/antimycotics, 2 mM L-glutamine and 10% fetal calf serum (Invitrogen GmbH Karlsruhe, Germany) in a CO2 incubator (5% CO2) at 37 °C. For the irradiation experiments, the cells were seeded into 12 well plates and grown to 100% confluency.Irradiation
For irradiation, the medium was replaced by phosphate-buffered saline (PBS). A quartz microplate of exactly the same dimensions was placed on top of the cell microplate and filled with ethanolic solutions of the UV filters (Fig. 1). The concentration of the solutions was normalized to an extinction of 800 at the respective absorption maximum (Fig. 2). The respective concentrations of the UV filter solutions were: 9.20 ppm (anisotriazine), 8.44 ppm (DHBB), 6.20 ppm (BMDM), 9.90 ppm (HRH22127), and 10.80 ppm (HRH21238). The amount of solution was adjusted to a pathlength of 1 cm. The microplate sandwich was covered with an accurately closing quartz lid and was then exposed to 20 J cm−2 UVA1 (Sellamed 24 000, Sellamed Geräte, Gevelsberg, Germany) (Fig. 3). The UVA1 lamp used in the present study is characterized by an UV spectrum ranging mainly between 340–400 nm as shown in Fig. 3. The relative percentages of radiant energy in the UVA1 (340–400 nm), UVA2 (320–340 nm), and visible (400–780 nm) ranges amounted to 96.9, 0.018 and 3.1%, respectively. No radiation in the infrared or UVB region was detectable.13 The UVA1 output was determined with a UVAMETER type II (Waldmann, Villingen-Schwenningen, Germany) and was found to be approximately 150 mW cm−2. After irradiation, the quartz plate was removed, the phosphate-buffered saline in the cell microplate was exchanged against culture medium and the cells were incubated in a CO2 incubator for 6 h (IL-1 and IL-6 determination) and 24 h (MMP-1 determination), respectively. The culture medium was removed, the cells were rinsed with phosphate-buffered saline and the whole plate was frozen in liquid nitrogen.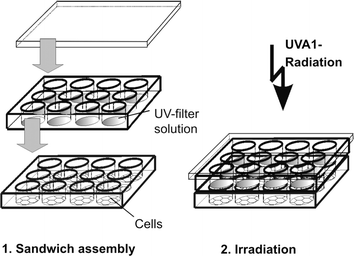 | ||
| Fig. 1 Experimental design used in the present study. | ||
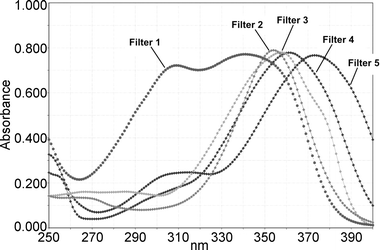 | ||
| Fig. 2 Absorption spectra of the different UVA1 filter solutions, normalized to E1%/1cm = 800. Filter 1 = anisotriazole; Filter 2 = DHBB; Filter 3 = BMDM; Filter 4 = HRH 22127; Filter 5 = HRH 21328. | ||
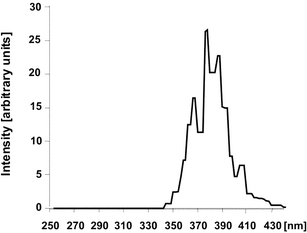 | ||
| Fig. 3 Spectral analysis of Sellamed 24 000 (reproduced from H. Stege, Technische Ausrüstung - Geräteübersicht, in Handbuch der dermatologischen Phototherapie und Photodiagnostik, ed. J. Krutmann and H. Hönigsmann, Springer, Berlin, Heidelberg, 1997, pp. 368–388). | ||
Isolation and quantification of MMP-1, IL-1 and IL-6 RNA
Total RNA was isolated using RNeasy Total RNA Kits (Qiagen, Hilden, Germany). The RNA concentration was determined via photometric measurement at 260 nm. Aliquots of total RNA (100 ng) were applied for cDNA-Synthesis using the Superscript™ III First-Strand synthesis system for RT-PCR (Invitrogen, Karlsruhe, Germany). For each gene, a specific primer pair was designed by Primer Express® 2.0 software (Applied Biosystems, Darmstadt, Germany) based on the cDNA sequence published as indicated. The following primer pairs were used: β-actin primer: sequence of Ponte et al.,14 and Vandekerckhove et al.;15 MMP1 primer: sequence of Whitham et al.;16 IL-1α primer: sequence of Nishida et al.;17 IL-6 primer: sequence of May et al..18 Three independent experiments were performed with 4 determinations each and the mean value of these was calculated. The PCR reactions were carried out on an Opticon 1 (MJ Research, Waltham, MA, USA) using SYBR® Green PCR Master Mix (Applied Biosystems, Darmstadt, Germany). For comparison of relative expression in real time PCR control cells and treated cells the 2-ΔΔCT method was used.19Results
The present experimental setup using a sandwich of a quartz microplate filled with ethanolic UV filter solutions placed on a cell microplate of the same dimensions was found to be easy to handle. It allowed a high number of measurements in a short time. We could not detect a significant difference in the volume of UV filter solution before (4.15 ± 0.021 mL) and after irradiation (4.09 ± 0.021 mL). Thus, evaporation was efficiently suppressed by the use of the accurately closing lid.To study the protective effect of UVA filters against UVA1-induced MMP-1 expression, we irradiated human dermal fibroblasts with 20 J cm−2 UVA1 through the quartz plate ± UVA filters as shown in Fig. 2. The dose of 20 J cm−2 is not cytotoxic as demonstrated previously and did not result in a reduction of the housekeeping gene β-actin in the irradiated cells (not shown).20 The cells were cultured for another 24 h as previous studies had demonstrated maximal expression of MMP-1 protein and mRNA at that time point.3,8,21,22 Then, the total RNA was isolated and MMP-1 mRNA was determined by real time RT-PCR.
MMP-1 mRNA was enhanced 8.1 ± 1.5 times at 24 h post irradiation. All UVA filters provided a statistically significant (p < 0.01) protection against UVA1-induced MMP-1 expression compared to the control without UV filter. However, the efficacy was dependent on the respective absorption properties (Fig. 4). The protection by the two filters with an absorption maximum >360 nm was better (HRH22127/filter 4 = 61% reduction of MMP-1, HRH21328/filter 5 = 58% reduction) than the protection of those filters with an absorption maximum below 360 nm (DHBB/filter 2 = −49%, BMDM/filter 3 = −48%, anisotriazine/filter 1 = −46%). Although the difference between the filters with an absorption maximum >360 nm and those filters with an absorption maximum <360 nm was not statistically significant (p > 0.05), there is a clear trend indicating that UVA1 light with wavelengths >360 nm is a more potent inducer of MMP-1 than UVA light with shorter wavelengths.
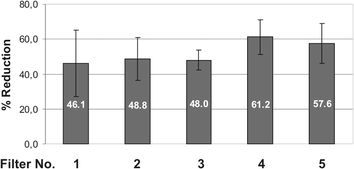 | ||
| Fig. 4 Reduction of UVA1-induced MMP-1 expression in human dermal fibroblasts by different UVA1 filters. The values are expressed as the % reduction relative to the control (= 0% reduction, pure ethanol). Mean values from 3 independent experiments with quadruple determinations each. p < 0.01 vs. control for all UVA filters. | ||
We next investigated whether HRH22127 and HRH21238 also protected against UVA1-induced expression of IL-1α, IL-1β and IL-6. Previous studies describing the interrelated autocrine cytokine loops showed maximal expression of IL-1α and IL-1β mRNA after 12 h and maximal expression of IL-6 after 6 h.3 As these experiments had been conducted using 30 J cm−2 UVA1 we also used this higher dose of UVA1 for irradiation. The UV doses used in these experiments did not cause cell lysis as shown by trypan blue exclusion assay, 3H-thymidine incorporation rate and the determination of cell number.3,4 The cytokines were expressed in a time-dependent manner with a maximum at 6 h after irradiation (IL-1α = 8.8 ± 3.6 fold, IL-6 = 24.9 ± 14 fold). While the amount of IL-6 detected after 6 h had declined by 28.5% after 12 h and was further reduced to −80% at 24 h, the amount of IL-1α decreased more rapidly (−72% at 12 h, −89% at 24 h) (Fig. 5). IL-1β was only poorly induced by UVA1-irradiation (2-fold after 6 h) and was therefore not included in the experiments. Although the induction of IL-1β was only 2-fold, this cytokine is supposed to be important for this particular cytokine loop as only the combined incubation with anti-IL-1α and anti-IL-1β antibodies during and after irradiation had been able to totally abrogate UVA-induced IL-6 bioactivity.3 Thus, 6 h was chosen as the time point for measurement of interleukin expression after UVA1 irradition. We found that both UVA1 filters efficiently suppressed the UVA1-induced IL-1α and IL-6 expression. Interestingly, the protection effect of HRH21238 (IL-1α = −65.8%, IL-6 = −71.8%) was superior to that of HRH22127 (IL-1α = −46.8%, IL-6 = −54.6%) (Fig. 6), which is underlined by: (i) the statistical significance of cytokine suppression relative to the irradiated control without UV filters (HRH21238: p < 0.05 in all experiments; HRH22127: p < 0.05 only in 2 out of 3 experiments; for both IL-1α and IL-6); (ii) the statistically significant difference (p = 0.0023) between the two UVA1 filters for IL-1α suppression; and (iii) the statistically significant difference between HRH21238 and HRH22127 in 2 out of 3 experiments (p = 0.02, 0.30, 0.02 for experiments 1, 2 and 3, respectively) for IL-6 suppression. These results suggest a significant role of wavelengths >370 nm for the induction of IL-1α and IL-6.
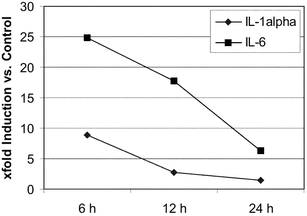 | ||
| Fig. 5 Course of UVA1-induced IL-1α and IL-6 expression in human dermal fibroblasts with time. The values are expressed as x-fold induction relative to the non-irradiated control. Mean values from 3 independent experiments with quadruple determinations each. | ||
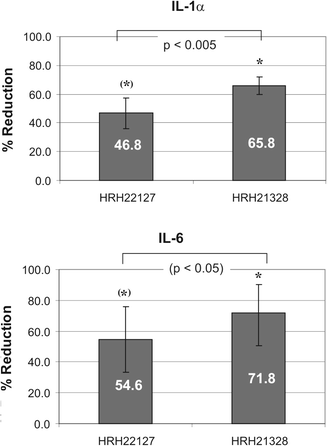 | ||
| Fig. 6 Reduction of UVA1-induced IL-1α and IL-6 expression in human dermal fibroblasts by HRH22127 and HRH21238. The values are expressed as the % reduction relative to the control (= 0% reduction, pure ethanol). Mean values from 3 independent experiments with quadruple determinations each. Symbols: * p < 0.05 vs. control in all 3 experiments; (*) p < 0.05 vs. control in 2 out of 3 experiments; IL-1α: p < 0.005 for HRH22127 and HRH21238 in all 3 experiments. IL-6: p < 0.05 for HRH22127 vs. HRH21238 in 2 of 3 experiments. | ||
Discussion
Photoaging is caused by repeated sun exposure and characterized by profound, detrimental alterations of the dermal connective tissue. The UVA1-triggered induction of MMP-1, which is further potentiated by UVA1-induced IL-1 and IL-6, is a key factor in cutaneous photodamage. In the present work we demonstrated that these events can be efficiently prevented by UVA1 filters significantly absorbing in the far UVA1 range (360–400 nm). Based on the absorption profile of the UVA1 filters investigated, our results point towards an important role for wavelengths >360 nm for MMP-1 induction, and for wavelengths >370 nm for the induction of IL-1α and IL-6.Our time course of cytokine induction is somewhat different from that described by Wlaschek et al..3 Whereas Wlaschek et al. found a maximum for IL-1α mRNA at 12 h post-irradiation and a maximum for IL-6 mRNA at 3–6 h post-irradiation,3 we found the highest levels of both interleukins at 6 h after UVA1 exposure. However, the induction level for MMP-1 in our experiments is comparable to that reported by Scharffetter et al. (5.2-fold induction 24 h after 30 J cm−2 UVA1),4 and by Polte and Tyrrell (7–10-fold induction of MMP-1 24 h after 25 J cm−2 UVA1).23
For the investigation of the photoprotective effect of UV filters in vivo, erythema is measured for UVB protection and persistent pigment darkening for UVA protection. However, these parameters only reflect part of the UV-induced reaction cascade in the skin. A more thorough investigation of UV-induced changes of selected biological parameters in vivo requires the extensive taking of biopsies. Thus, in vitro methods are preferred. Most UV filters are highly hydrophobic, and therefore can not be added directly to the cell culture medium. To overcome this problem, in some studies sunscreen solutions or sun formulations were applied on a quartz slide or in between two quartz slides.24–26 The slides were placed on top of cell culture dishes and the whole sandwich was then irradiated. However, these methods suffer from several problems: (i) danger of evaporation; (ii) inhomogeneity of the UV filter film; (iii) extensive handling; (iv) low throughput. The same problems occur if three-dimensional skin equivalents or ex vivo skin explants are used.
To understand the biological consequences of sun exposure and to develop suitable protection strategies, efficient in vitro screening systems are required. In the present study we used a sandwich of a quartz microplate filled with ethanolic UV filter solutions placed on a cell microplate of the same dimensions. The whole sandwich was irradiated and the cells analyzed for the desired biological endpoints. This experimental set-up is easy to handle and allows for high throughput screening. The microplate sandwich method enables multiple applications. In particular, UV filters can be rapidly screened for their properties in photobiological protection. Moreover, by the aid of sunscreens with different absorption profiles it is possible to approximate the action spectra of single biological endpoints. This knowledge enables the targeted development of new sunscreens and possibly also of sunscreen formulations with an optimal photoprotective profile. In addition, a parallel investigation of sunscreens (in the quartz plate) and of active ingredients such as e.g. antioxidants, antiirritants or MMP-1 inhibitors (in the cell plate) can be realized.
The aim of the present study was the investigation of the net effects of individual UVA filters on MMP-1 and cytokine inhibition. We have not yet tested the suitability of the present method for the investigation of sunscreen formulations. This will be the subject of a follow-up study. However, the results obtained with the ethanolic UV filter solutions are expected to be analogous to sunscreen formulations since the relative differences in the different absorption properties of the UV filters used here are expected to be comparable to finished sunscreen formulations. This is because any solvatochromic effects on the absorbance profiles of each UV filter studied can be anticipated to be similar due to the electron charge distribution of the excited states of the molecules being similar. Each molecule has an electron donating group coupled to one end of the chromophore with an electron accepting group at the other end, so the relative efficacy would be maintained.30
To provide standardized conditions, we normalized the concentrations of the UVA filter solutions to a maximum specific extinction (E1%/1cm) of 800. These correlated with a critical wavelength (CW) of 365, 373, 374, 384, and 391 nm, respectively, for filters 1–5. The CW is somewhat higher for filters 1–3 (368, 374, 378 nm) if calculated based on equally concentrated solutions. Nevertheless, for both calculations the same conclusion applies: UV filters exhibiting a CW >380 nm protect best against UVA1 induced MMP-1, IL-1 and IL-6 induction.
To date, only poor information on the action spectrum of dermal photoaging is available. The action spectrum for UVA-induced sagging in mouse skin has a maximum at 340 nm.27 In contrast, the wavelength maximum for induction of elastosis in mouse skin was found to be in the UVB range.28,29 However, as human skin is thicker than mouse skin, longer UV wavelengths might be required to reach biologically effective doses in human skin relative to mouse skin. This hypothesis is supported by the study of Lavker and Kaidbey on the wavelength dependence for UVA-induced damage in human skin.31 They found that the action spectrum for UVA-induced chronic dermal damage is broad, extending up to 400 nm. Wavelengths of 335, 345, and 360 nm were equally effective in inducing lysozyme deposition on elastin fibers and dermal inflammatory infiltrates which are well documented hallmarks of photodamage.32–35 Our observation of a significant role for UVA1 irradiation in cytokine and collagenase induction fits to the action spectrum of lysozyme activation and dermal infiltration described by Lavker and Kaidbey, adding more detailed information on the wavelength range of 360–380 nm. Thus, the microplate sandwich system presented here could serve as a valuable tool to study the action spectrum of several other biological endpoints related to photoaging in vitro, such as e.g. induction of collagen crosslinking, heme oxygenase-1, stress genes or damage to mitochondrial and/or nuclear DNA.
Taken together, these results suggest that an optimal protection against UVA-induced photoaging (wrinkling) is achieved by application of UV filters with a maximum absorption at ≥360 nm and a critical wavelength of >380 nm. In addition, the method used in this study is rapid and easy to handle, thus enabling a high-throughput screening of UV filters for their cellular protection properties in vitro. Moreover, by the use of the microplate sandwich method it will be possible to identify the optimal protecting absorption profile against selected UV-induced biological endpoints via the targeted investigation of different UV filters.
Acknowledgements
Excellent technical assistance by Heidi Brenden is gratefully acknowledged.References
- H. R. Griffiths, P. Mistry, K. E. Herbert and J. Lunec, Molecular and cellular effects of light-induced genotoxicity, Crit. Rev. Clin. Lab. Sci., 1998, 35, 189–237 Search PubMed.
- A. Sarasin, The molecular pathways of ultraviolet-induced carcinogenesis, Mutat. Res., 1999, 428, 5–10 CrossRef CAS.
- M. Wlaschek, G. Heinen, A. Poswig, A. Schwarz, T. Krieg and K. Scharffetter-Kochanek, UVA-induced autocrine stimulation of fibroblast-derived collagenase/MMP-1 by interrelated loops of interleukin-1 and interleukin-6, Photochem. Photobiol., 1994, 59, 550–556 CrossRef CAS.
- K. Scharffetter, M. Wlaschek, A. Hogg, K. Bolsen, A. Schothorst, G. Goerz, T. Krieg and G. Plewig, UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo, Arch. Dermatol. Res., 1991, 283, 506–511 CrossRef CAS.
- C. Kut, W. Hornebeck, N. Groult, G. Redzinack, G. Godeau and B. Pellat, Influence of successive and combined ultraviolet A and B irradiations on matrix metalloproteinases produced by human dermal fibroblasts in culture, Cell Biol. Int., 1997, 21, 347–352 CrossRef CAS.
- M. Berneburg, S. Grether-Beck, V. Kurten, T. Ruzicka, K. Briviba, H. Sies and J. Krutmann, Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion, J. Biol. Chem., 1999, 274, 15345–15349 CrossRef CAS.
- M. Yaar and B. A. Gilchrest, in Photodamage, ed. B. Gilchrest, Blackwell Science, New York, 1995, pp. 123-135 Search PubMed.
- M. Wlaschek, K. Bolsen, G. Herrmann, A. Schwarz, F. Wilmroth, P. A. Heinrich, G. Goerz and K. Scharffetter-Kochanek, UVA-induced autocrine stimulation of fibroblast-derived collagenase by IL-6: a possible mechanism in dermal photodamage?, J. Invest. Dermatol., 1993, 101, 164–168 CrossRef CAS.
- B. L. Diffey, P. R. Tanner, P. J. Matts and J. F. Nash, In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products, J. Am. Acad. Dermatol., 2000, 43, 1024–1035 CrossRef CAS.
- A. Fourtanier, A. Gueniche, D. Compan, S. L. Walker and A. R. Young, Improved protection against solar-simulated radiation-induced immunosuppression by a sunscreen with enhanced ultraviolet A protection, J. Invest. Dermatol., 2000, 114, 620–627 CrossRef CAS.
- S. Seite, D. Moyal, M. P. Verdier, C. Hourseau and A. Fourtanier, Accumulated p53 protein and UVA protection level of sunscreens, Photodermatol. Photoimmunol. Photomed., 2000, 16, 3–9 CrossRef CAS.
- C. Augustin, C. Collombel and O. Damour, Measurements of the protective effect of topically applied sunscreeens using in vitro three-dimensional dermal and skin equivalents, Photochem. Photobiol., 1997, 66, 853–859 CrossRef CAS.
- S. Tzaneva, A. Seeber, M. Schwaiger, H. Hönigsmann and A. Tanew, High-dose versus medium-dose UVA1 phototherapy for patients with severe generalized atopic dermatitis, J. Am. Acad. Dermatol., 2001, 45, 503–507 CrossRef CAS.
- P. Ponte, S. Y. Ng, J. Engel, P. Gunning and L. Kedes, Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA, Nucleic Acids Res., 1984, 12, 1687–1696 CrossRef CAS.
- J. Vandekerckhove and K. Weber, Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 1106–1110 CAS.
- S. E. Whitham, G. Murphy, P. Angel, H. J. Rahmsdorf, B. Smith, A. Lyons, T. J. R. Harris, J. J. Reynolds, P. Herrlich and A. J. P. Docherty, Comparison of human stromelysin and collagenase by cloning and sequence analysis, Biochem. J., 1986, 240, 913–916 CAS.
- T. Nishida, N. Nishino, M. Takano, K. Kawai, K. Bando, Y. Masui, S. Nakai and Y. Hirai, cDNA cloning of the IL-1 alpha and IL-1 beta from mRNA of U937 cell line, Biochem. Biophys. Res. Commun., 1987, 143, 345–352 CrossRef CAS.
- L. T. May, D. C. Helfgott and P. B. Sehgal, Anti-β-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the β2 interferon involved, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 8957–8961 CAS.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method, Methods, 2001, 25, 402–408 Search PubMed.
- J. Wenk, P. Brenneisen, M. Wlaschek, A. Poswig, K. Briviba, T. D. Oberley and K. Scharffetter-Kochanek, Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1, J. Biol. Chem., 1999, 274, 25869–25876 CrossRef CAS.
- M. J. Petersen, C. Hansen and S. Craig, Ultraviolet A irradiation stimulates collagenase production in cultured human fibroblasts, J. Invest. Dermatol., 1992, 99, 440–444 CrossRef CAS.
- M. Wlaschek, K. Briviba, P. Stricklin, H. Sies and K. Scharffetter-Kochanek, Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase, J. Invest. Dermatol., 1995, 104, 194–198 CrossRef CAS.
- T. Polte and M. Tyrell, Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression, Free Radical Biol. Med., 2004, 36, 1566–1574 CrossRef CAS.
- S. Jean, M. De Meo, A. S. Sabatier, M. Laget, J. C. Hubaud, P. Verrando and G. Dumenil, Evaluation of sunscreen protection in human melanocytes exposed to UVA or UVB irradiation using the alkaline comet assay, Photochem. Photobiol., 2001, 74, 417–423 CrossRef CAS.
- P. Reinhardt, M. Cybulski, J. P. McNamee, J. R. McLean, W. Gorman and Y. Deslauriers, Protection from solar simulated radiation-induced DNA damage in cultured human fibroblasts by three commercially available sunscreens, Can. J. Physiol. Pharmacol., 2003, 81, 690–695 CrossRef CAS.
- L. Marrot, J. P. Belaidi and J. R. Meunier, Comet assay combined with p53 detection as a sensitive approach for DNA photoprotection assessment in vitro, Exp. Dermatol., 2002, 11(Suppl. 1), 33–36 CrossRef CAS.
- D. L. Bissett, D. P. Hannon and T. V. Orr, Wavelength dependence of histological, physical, and visible changes in chronically UV-irradiated hairless mouse skin, Photochem. Photobiol., 1989, 50, 763–769 CrossRef CAS.
- L. H. Kligman and R. M. Sayre, An action spectrum for ultraviolet induced elastosis in hairless mice: quantification of elastosis by image analysis, Photochem. Photobiol., 1991, 53, 237–242 CrossRef CAS.
- H. C. Wulf, T. Poulsen, R. E. Davies and F. Urbach, Narrow-band UV radiation and induction of dermal elastosis and skin cancer, Photodermatology, 1989, 6, 44–51 CAS.
- N. A. Shaath, in Sunscreens: Development, Evaluation and Regulatroy Aspects, ed. N. J. Lowe, N. A. Shaath and M. A. Pathak, Marcel Dekker, New York, 2nd edn, 1997, pp. 263–283 Search PubMed.
- R. Lavker and K. Kaidbey, The spectral dependence for UVA-induced cumulative damage in human skin, J. Invest. Dermatol., 1997, 108, 17–21 CrossRef CAS.
- H. Suwabe, A. Serizawa, H. Kajiwara, M. Ohkido and Y. Tsutsumi, Degenerative processes of elastic fibers in sun-protected and sun-exposed skin: immunoelectron microscopic observation of elastin, fibrillin-1, amyloid P component, lysozyme and alpha1-antitrypsin, Pathol. Int., 1999, 49, 391–402 CrossRef CAS.
- S. Seite, A. Colige, P. Piquemal-Vivenot, C. Montastier, A. Fourtanier, C. Lapiere and B. Nusgens, A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging, Photodermatol. Photoimmunol. Photomed., 2000, 16, 147–155 CrossRef CAS.
- R. M. Lavker, G. F. Gerberick, D. Veres, C. J. Irwin and K. H. Kaidbey, Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin, J. Am. Acad. Dermatol., 1995, 32, 53–62 CrossRef CAS.
- B. A. Gilchrest, N. A. Soter, J. L. Hawk, R. M. Barr, A. K. Black, C. N. Hensby, A. I. Mallet, M. W. Greaves and J. A. Parrish, Histologic changes associated with ultraviolet A-induced erythema in normal human skin, J. Am. Acad. Dermatol., 1983, 9, 213–219 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |
