Biochemistry of human skin—our brain on the outside
Desmond J.
Tobin
Medical Biosciences, School of Life Sciences, University of Bradford, Bradford, West Yorkshire, England, UK BD7 1DP. E-mail: dtobin@bradford.ac.uk; Fax: 01274 3909742; Tel: 01274 233585
First published on 26th October 2005
Abstract
The skin, our body's largest organ, is located at the interface between the external and internal environments, and so is strategically placed to provide not only a barrier against a range of noxious stressors (UV radiation, mechanical, chemical and biological insults) but also to act as the periphery's ‘sensing’ system. Recent developments suggest that this organ is much more critical to maintaining body homeostasis than previously thought. This tutorial review introduces the reader to some of the biochemistry that underpins the skin's enormous multi-functionality.
 Des Tobin | Des Tobin obtained a primary degree from the National University of Ireland – Maynooth and a PhD from the University of London, UK (St. Thomas's Hospital Medical School) in 1991. He worked as a Post-doctoral Fellow in the Department of Dermatology, New York University Medical School before joining the junior faculty there. On his return to Europe in 1996, he took up a lecturing position in the Department of Biomedical Sciences at the University of Bradford where he is now Professor of Cell Biology. |
1 Introduction
The last 20 years has seen enormous growth in our knowledge of skin function. Several new sub-specialities of cutaneous biology have emerged, not least of which is cutaneous neuro-endocrinology. The latter positions the skin as sensor of the periphery and has prompted some to call the skin our ‘brain on the outside’.1 A true biologic universe, the skin incorporates all major support systems; blood, muscle and innervation as well as roles in immuno-competence, psycho-emotion, ultraviolet radiation sensing, endocrine function etc. Together, these help to maintain the homeostasis of the mammalian body. When a giant of dermatology was asked recently to comment on the ‘true’ function of skin, he felt inclined to invert the question to “Is there anything that the skin can't do?2 With this in mind, I begin this brief tutorial review on skin with the aim of highlighting recent biochemical elucidations on skin functioning that are likely to offer promise for human health. Explanations/definitions of some unavoidable jargon appear within square brackets.In the 1940s the immunologists Billingham, Medawar and Brent provided the first real demonstration of a cellular basis for immunity with their work on the fate of skin grafts.3 However, these researchers were not particularly interested in the skin per se, and it is only in the last couple of decades that the skin, as a distinct organ, has been more fully appreciated as the major transducer of signals from the environment to the individual, and between individuals. Particularly important here is the recent identification of the skin and its appendages as both a source and target of neuro-transmitters, -hormones and -peptides thought previously to be the domain of the central nervous system. While the ‘new’ technologies of molecular biology, proteomics and genomics etc. have been invaluable in unravelling the complexities of skin function, much of our knowledge still remains somewhat phenomenologic. This is based on knowledge of particular genotypes [genetic constitution] and/or phenotypes [visible/measurable physical and biochemical characteristics—the product of the genotype interacting with environmental factors]. While the ‘biology’ of the skin as a ‘signal’ or ‘biosensor’ has grown hugely over the last decade, the ‘chemistry’ side of cutaneous sciences has largely been limited to cosmetic chemistry. Thus, a renaissance in cutaneous biochemistry is now required for the optimal understanding of all this new information. Indeed, the last significant treatment of biochemistry of skin was the two-volume work by Lowell A. Goldsmith in 1991.4
2 Components of human skin5
Adult human skin extends to approximately 2 m2 in area, is around 2.5 mm thick on average and has an average density of 1.1. Together these provide for a 5–6 kg mass value for skin or to put it another way skin constitutes an impressive 6% of our total body weight. As such the skin exceeds all other organs in total mass, if we exclude the muscle, bone, adipose and blood systems from this limited definition of ‘organ’.6 The skin invests the body to provide a vast physical barrier at the interface with the external environment and is designed to protect us against a range of insults including: desiccant (temperature, electrolyte/fluid balance), mechanical, chemical and microbial. Further protection is provided by the ultraviolet radiation (UVR)-absorbing pigmentation system and the complex immuno-regulatory sentinel networks, which sense tissue micro-environments for foreign or abnormally expressed components. It is only by thinking of the skin in holistic terms can we accommodate, and then tackle, the difficulties in modern clinical dermatology, dermato-pathology, and dermato-pharmacology that will hopefully guide us to development of targets for therapeutic intervention.Conventionally, the skin is described as consisting of two broad tissue types (Fig. 1); the epidermis – an external stratified, non-vascularized, epithelium of between 75 and 150 µm (up to 600 µm thick on palms/soles), and an underlying connective tissue called the dermis – consisting of mixture of fibroblasts producing dense fibrous/elastic components and the erroneously termed ‘ground’ substance. The dermis may be up to 4 mm thick (e.g. adult back) but is usually less than 2 mm and houses many of the skin's ‘business centers’ including; its vascular, neural and lymphatic systems and its multiple accessory appendages (Fig. 2). The latter include its excretory and secretary glands (Sebaceous, Eccrine and Apocrine glands), its keratinizing structures (Hair follicles and Nails), and its sensory nerve receptors of Meissner's corpuscles (touch), Pacinian corpuscles (pressure), Pilo-Ruffini corpuscles (mechanoreceptors), free terminals, hair follicle endings etc. There is considerable variation in the presence and density of these appendages between different body sites. Finally, anatomists include a third skin layer, the sub-cutis or hypodermis consisting of fatty connective tissue that connects the dermis to underlying skeletal components. Interested readers keen on learning more about the cell biology and physiology of skin can consult specialized texts.5
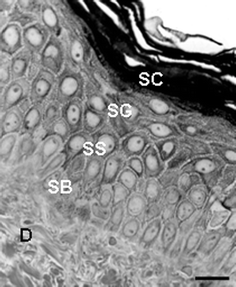 | ||
| Fig. 1 High-resolution light microscopy view of a portion of normal human skin showing epidermis and dermis (D). SC, S. corneum; SG, S. granulosum; SS, S. spinosum; SB, S. basale. Scale bar = 30 µM. | ||
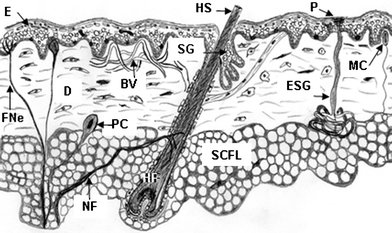 | ||
| Fig. 2 Cartoon of normal human skin showing epidermis (E), dermis (D), subcutaneous fat layer (SCFL), and skin appendages including hair follicle (HF) with hair shaft (HS), sebaceous gland (SG), eccrine sweat gland (ESG), Meissner's corpuscle (MC), Pacinian corpuscle (PC), blood vessels (BV), sweat gland pore (P), nerve fibre (NF), free nerve ending (FNe). | ||
2.1 Epidermis
The epidermis is a continually keratinizing or cornifying [hardening] stratified epithelium terminating at muco-cutaneous junctions (e.g. mouth). Pores of glands and hair follicles disrupt it. This outer layer consists of four major ‘strata’ composed principally of keratin-producing cells called keratinocytes. The outermost Stratum Corneum is a cornified layer of 15–30 sheets of non-viable, but biochemically-active cells called corneocytes (Fig. 3). The next, inner, layer is the S. granulosum, a 3–5 sheets granular layer of non-dividing keratinocytes producing granules of a protein called keratinohyalin (Fig. 3). These cells flatten as dividing cells below progressively push them to the skin surface. At the same time their cell organelles and nuclei break down and their cell membranes become increasingly impermeable. This layer is followed by the S. spinosum, or ‘spiny’ layer (Fig. 4), containing 8–10 sheets of keratinocytes with some limited capacity for cell division. Also found here are bone marrow-derived sentinel cells of the immune system called Langerhans Cells, which scour the skin for evidence of entry by ‘foreign’ entities. The basal or dividing layer of the epidermis is called the S. basale or S. germinativum (Fig. 4). These cells are attached to a non-cellular basement membrane that separates the epidermis from dermis. In addition to the differentiating (i.e., maturing/aging) keratinocytes, the S. basale also houses other keratinocytes with stem cell-like properties, the enigmatic Merkel cell (with apparent sensory functions) and melanocytes. The latter are important pigment (melanin)-producing cells that help protect us against the damaging effects of UVR radiation (UVR, see below).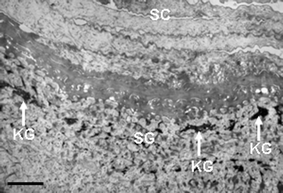 | ||
| Fig. 3 Transmission electron microscopy view of the upper epidermis of normal human skin showing the S. corneum (SC) and S. granulosum (SG). Note the presence of dark-staining keratohyalin granules (KG). Scale bar = 1 µM. | ||
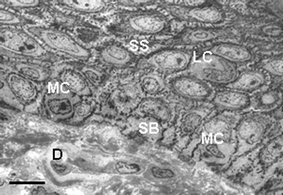 | ||
| Fig. 4 Transmission electron microscopy view of the lower epidermis of normal human skin showing keratinocytes of the S. spinosum (SS) with additional Langerhans cells (LC), and keratinocytes of the S. basale (SB) with additional melanocytes (MC). Scale bar = 20 µM. | ||
Merkel cells: It is worthwhile spending a brief moment to comment on one of the skin's most enigmatic cell types—The Merkel Cell. First described in 1875 by F. S. Merkel, these large and pale-staining cells are located in the basal layer of mammalian epidermis where they function as slow-adapting mechanoreceptors via synapse-like contacts with enlarged terminal endings of myelinated nerve fibres. They tend to be concentrated around whisker follicles in several mammalian species, and in humans are rich in eccrine glandular ridges of glabrous skin and within belt-like clusters of hair follicles. While similar-appearing cells are distributed elsewhere in the skin these do not function as mechanoreceptors but are likely part of the skin neuroendocrine system, and may even be the origin of a highly aggressive skin cancer called Merkel cell carcinoma.7,8
However, recent results from chick/quail chimeras and from double transgenic mice have indicated that these cells are derived from the neural crest. This is despite their prominent epithelial cytoskeletal features including both desmosomal-type intercellular junctions (which attach these cells to one another and to neighboring keratinocytes) and their very specific CK20 keratin. However, these epithelial features of Merkel cells facilitate their role in maintaining the 3-D structure of the epidermis. Other characteristic features of Merkel cells include the presence of numerous osmiophilic dense-core cytoplasmic granules containing neuropeptides, some of which are likely to act as neurotransmitters. Mechanical stimuli result in an influx of Ca2+ ions in Merkel cells to trigger release of neurotransmitter, including most probably glutamate.7,8
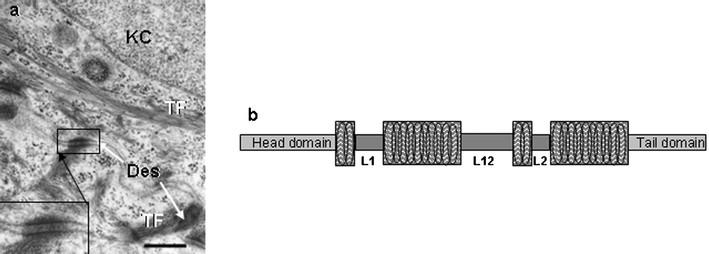 | ||
| Fig. 5 a) Transmission electron microscopy view of part of two adjacent keratinocytes (KC) of the S. spinosum in normal human skin. Note presence of tonofilaments (TF) and intercellular junctions called desmosomes (Des, inset). Scale bar = 0.4 µM. b) Cartoon of a keratin intermediate filament consisting of a head and tail domain separated by 4 coiled α-helical regions interspersed with linker regions (L1, L2, L12). | ||
As a family, KIFs are elongated α-helix-enriched proteins that form dynamic coiled-coils (Fig. 5b), which are fashioned into filament collections called tonofilaments (Fig. 5a). The latter are soluble only in denaturants like urea and sodium dodecyl sulfate, and reducing reagents like mercaptoethanol and dithiothreitol. High-resolution two-dimensional gel electrophoresis and use of monoclonal antibodies that probe specific keratin types have permitted the cataloguing of human epidermal keratins.11 These studies showed that epithelial keratins could be grouped as being neutral-basic or acidic in charge. Subsequently, amino acid sequencing indicated that acidic keratins shared sequence homology and were called Type I, while neutral-basic keratins were similar and so called Type II. Each cell/tissue type expresses at least one Type I keratin together with its preferred Type II keratin partner—the ‘keratin pairs’ concept. Thus, KIFs in each epithelial cell type are obligate hetero-polymers and while both chains generally are expressed simultaneously one partner may be expressed before the other. Indeed, this order can be reversed under hormonal stimulation in some tissues. Similarly, inhibition of expression of one partner chain results in inhibition of the partner chain. K5 and K14 are associated with cell proliferation in the epidermis, while commitment to terminal keratinocyte differentiation (i.e. maturation) is associated with expression of K1 & 2 and K10 & K11.
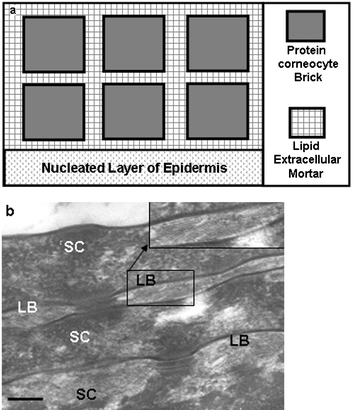 | ||
| Fig. 6 a) Schematic of the ‘brick and mortar’ view of the organization of the S. corneum, where the brick is represented by the protein-rich, lipid-poor, corneocyte and the ‘mortar’ by the lipid-rich, protein-poor, extra-cellular material. b) Transmission electron microscopy high-power view of the S. corneum (SC) of normal human skin showing the protein-rich corneocyte (SC) separated by the lipid-rich lamellar body/granules (LB). Scale bar = 0.15 µM. | ||
Barrier function in human epidermis depends on transglutaminase (TGase)-mediated cross-linking of structural proteins and lipids during the terminal stages of keratinocyte differentiation.14 During this process these so-called ‘biological glues’ catalyse the post-translational modification of proteins by forming isopeptide bonds either through protein cross-linking via epsilon-(gamma-glutamyl)lysine bonds or through the incorporation of primary amines at selected peptide-bound glutamine residues. Such cross-linking not only yields protein products of high molecular mass, but these proteins are highly resistant to mechanical perturbation and proteolysis. Several mutations in genes that encode for the barrier's structural components (e.g. CE proteins and lipids) and its enzymes and lipid processing can result in skin disease. In addition, recent evidence also shows that epidermal differentiation also involves the maintenance of functional protease/anti-protease systems in both the epidermis and hair follicle. Associated pathologies result from mutations therein, e.g. in SPINK5 serine proteinase inhibitor (Netherton syndrome), cathepsin C mutations (Papillon–Lefevre syndrome), and cathepsin L deficiency (furless mice).15,16
The quality of the S. corneum barrier depends on the presence of equimolar amounts of ceramides, cholesterol and free fatty acids. Changes in the concentrations of any of these can affect barrier quality. Aged and photo-aged skin exhibit a cholesterol-dominant barrier, while atopic dermatitis is associated with ceremide-dominance and a dominance of free fatty acids is associated with psoriasis.2
Ceramides account for approximately 50% of S. corneum lipids followed by cholesterol and free fatty acids. Inhibiting key enzymes involved in the synthesis of these lipids can disturb barrier permeability. Both Ca2+ and K+ are important regulators of barrier function, and synergistically inhibit barrier repair via blocking the return of lipids to the S. corneum. Thus, barrier repair may be initiated by loss of these ions during water influx in the damaged epidermis. It also appears that acid pH is required for maintenance of epidermal permeability barrier homeostasis, perhaps by providing optimal pH for the enzymes involved in extracellular processing bilayer lipids. The precise origin of acidic pH in this system is still unclear, but may derive from exogenous sources e.g., lactic acid in sweat, free fatty acids in sebum and metabolites of skin microflora. Comfortingly, ‘friendly’ skin microbes (e.g., micrococci) tend to like the acidic pH while ‘enemy’ microbes (e.g., staphylococci) prefer neutral/basic conditions. A recent report presented the tantalizing possibility that albumin [so-called blood plasma protein] may be synthesized locally in the epidermis, where it can bind and transport unsaturated fatty acids, Ca2+, tryptophan, heavy metals (like copper and nickel), vitamins B6 and B12, as well as folic acid.17 The full implications of this exciting discovery await further determination.
Skin fatty acids. The permeability of human skin is largely based on the quality and quantity of lamellar lipid domains packed between corneocytes of the S. Corneum. Endogenously-produced fatty acids can be found not only in epidermal cell membranes, but also in the lipid located between the layers of corneocytes in the S. corneum, and in the so-called hydro lipid film at the skin surface. These biomolecules are crucial contributors to the structure–function relationship of the skin epidermis18. However, fatty acids are not only involved in the end stages of epidermal cell life in the S. corneum, but also participate in signalling events during keratinocyte proliferation and differentiation deep in the epidermis and even dermis, and are also implicated in the skin immune response (see below).
Perturbation in skin fatty acid status can lead to disease. For example, atopic dermatitis [eczema due to acute or chronic inflammatory reactions with a genetic basis] is associated with abnormally reduced omega-6 fatty acid levels (and decreased ceramide content). The hallmark of perturbed barrier function i.e., increased water loss from skin surface, is also seen here. There is evidence that UV irradiation of skin can lead to increased lipid levels in the stratum corneum and this has been associated with improved barrier performance. Conversely, both photo-aged and chronologically-aged epidermis exhibit abnormal barrier homeostasis and exhibit not only an overall reduction in stratum corneum lipids but also a dramatic fall in the rate of cholesterol synthesis.19
Fatty acids in the epidermis can also be derived from exogenous sources including diet.20 Indeed, the skin is an active metabolizer of polyunsaturated fatty acids (PUFA). Diets deficient in some of these PUFA e.g. linoleic acid, can result in both increased TEWL and scaly skin. Arachidonic acid, a 20-carbon PUFA can be metabolized by either the cyclooxygenase or lipoxygenase pathways in skin with resultant pathway specific metabolites including prostaglandins and hydroxyeicosatetraenoic acids. Some of these metabolites can interact with signalling systems in the proliferating and differentiating epidermal cells, e.g. modulation of protein kinase C, nuclear MAP-kinase and apoptosis events.20
Indeed, most drugs used in dermatologic practice today are either substrates or inducers, or inhibitors of the CYP enzyme family, and components in cosmetics, toiletries, and health-care products commonly serve as substrates for CYP. Of special relevance to skin is the role CYPs play in mediating photosensitivity, and reactions to sulfonamides, tetracyclines etc.23 Moreover, retinoids used as potent therapeutic agents in skin disease are also metabolized in skin via a CYP-dependent hydroxylation.23 Improved understanding of how the skin handles xenobiotics is likely to come from continuing development in pharmacogenetics and pharmacogenomics. Indeed, the observed pharmacogenetic polymorphisms in members of the CYP family are likely to explain inter-individual responses to drug therapy and determine individual absorption, disposition, metabolism, and excretion of xenobiotics and drugs and so aid rational drug design.24
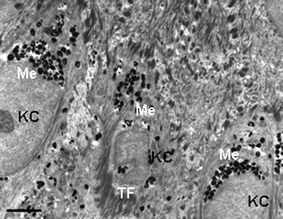 | ||
| Fig. 7 Transmission electron microscopy view of the lower epidermis of normal human skin showing the supra-basal keratinocytes (KC) containing multiple melanin granules clustered predominantly above the KC cell nucleus (Me). Note tonofilament clumps (TF). Scale bar = 2.5 µM. | ||
The principal marker for keratinocyte/epidermal differentiation is the expression of particular keratin pairs. Of the more than 30 keratins currently known to science, the majority of these are associated with the skin and its appendages. Broadly speaking, proliferative basal keratinocytes express K5 and K14, while keratinocytes in the early stages of maturation/differentiating switch to K1 and K10. Moreover, K6 and K16 are expressed in epithelial cells of the outer root sheath of hair follicles, nail beds, and epithelium of oral mucosa, and the supra-basal expression of K6 and K16 can occur when epidermis hyper-proliferates, such as in psoriasis and wound-healing express.9 One of the great recent advances in cutaneous science has been the identification of keratinocyte ‘stem’ cells. It now appears that ‘pluri-potent’ stem cells [cells that are so immature that they have the potential to give rise to keratinocytes of the hair follicle, sebaceous gland and epidermis] reside principally in the hair follicle rather than the epidermis itself.26–28 Ca2+ appears to play an important role in determining the controls for moving epidermal cells from the stem compartment to the so-called ‘transit-amplifying’ compartment. A particularly crucial cell-cycle inhibitor protein called p21 also controls the expression of late differentiation keratinocyte markers like filaggrin (a KIF-aggregating protein) and trans-glutaminase (an enzyme that cross-links/assembles proteins in the S. corneum; see above).
The pivotal role for Ca2+ in epidermal differentiation is reflected by a 4-fold increase in extracellular Ca2+ from basal layer to S. corneum. Moreover, addition of Ca2+ to keratinocytes in vitro arrests the growth in proliferating cells, while also inducing the expression of differentiation markers like K1/K2, fillagrin, involucrin and loricrin. Several signal transduction pathways [mechanisms by which extracellular signals are processed by cells to yield an intracellular effect] regulate keratinocyte differentiation. In addition to Ca2+, keratinocyte differentiation can be modulated by hormones and vitamins e.g., retinoic acid (vitamin A/retinol from diet), vitamin D3, thyroid hormone and steroid hormones. These act as ‘ligands’ that bind ‘receptors’ [as a key engages with its specific lock] that are expressed in cell nuclei. Engagement of nuclear receptors thereafter influences the expression of multiple genes involved in cell differentiation. The skin has nuclear receptors for several steroid hormones, including glucocorticoids, estrogen, androgen and progesterone, and can metabolise/transform active sex steroids from adrenal C19 precursor steroid in the skin itself.29
A plethora of interacting signalling systems finely tune/regulate keratinocyte function, particularly their intercellular adhesion and the communication that results from such intimate cell–cell contacts. Ca2+ is again a key factor here, as it controls the expression of several keratinocyte intercellular connections including desmosomal, adherens, gap and tight junctions. These junctions are more than ‘spot welds’ or ‘belts’ that hold cells in close apposition. They are also important conduits for communication, and the increase in Ca2+ from basal to upper layers facilitates an increase in desmosome size and number.
A myriad of other proteins emerge onto the scene as the keratinocyte embarks on its differentiation-led journey. Major ones include involucrin—a large α-helical flexible rod-like protein with 10-amino acid repeats containing 3 glutamines. The latter can be extensively cross-linked to other proteins by the enzyme transglutaminase, to render these protein complexes insoluble. High Ca2+ levels are required for the synthesis of involucrin in keratinocytes and also for their detachment from the basement membrane and withdrawal from the cell cycle. While the biochemistry of keratinocyte differentiation cannot proceed without Ca2+, this cation does not induce the transition of cells in the S. spinosum to the S. granulosum. Rather, this is mediated by protein kinase C via suppression of K1 and K10 gene expression, which is associated with the induction of the late differentiation markers loricrin and profillagrin.30 When keratinocytes mature to become members of the S. granulosum their most prominent feature is their protein-rich and lipid-rich granules31 (Fig. 3, 6b). The former, called keratohyalin granules, are irregularly shaped profilaggrin-containing granules that coalesce in the S. granulosum (Fig. 3) and thereafter disperse in the S. corneum upon dephosphorylation and subsequent maturation via proteolysis to filaggrin. The latter are lipid-rich lamellar (or Odland) granules (Fig. 6b), also present in these upper epidermal layers, which exist as lipid bilayers that fuse with the cell membranes to contribute lipids to the extracellular space of the S. corneum (see 2.1.2 above). These lipids form the sheets of the lipid permeability barrier of the epidermis.
Keratinocyte differentiation is not possible if basal keratinocytes are first not displaced from their hemi-desmosome linked ‘seat’ on the basement membrane that separates epidermis from underlying dermis (Fig. 8). One particularly important attachment combination is binding via Ca2+-dependent integrins. In particular, integrin α5β1 is the receptor for the extracellular matrix molecule called fibronectin. Other basement membrane components are the laminins and collagens (IV and VII), which regulate how keratinocytes migrate on basement membrane (very important during wound healing). Moreover, migrating keratinocytes produce a range of matrix metalloproteinases (MMPs) [zinc-dependent matrix-degrading enzymes] that in turn can be inhibited by tissue-derived inhibitors of metalloproteinases (TIMPs). Modulating the expression of MMPs and TIMPs by lowering oxygen and pH levels (like conditions found under occlusive dressings) also facilitates keratinocyte migration.32
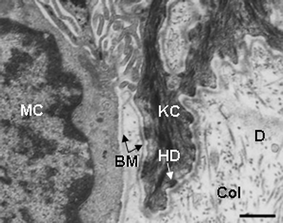 | ||
| Fig. 8 Transmission electron microscopy view of the lower epidermis of normal human skin showing the basement membrane zone separating the S. basale with keratinocytes (KC) and melanocytes (MC) and the collagen (Col)-rich dermis (D). Note the attachment of the KC to the basement membrane (BM) via hemi-desmosomes (HD). Scale bar = 0.6 µM | ||
2.2 The dermis
The biochemical complexity of the dermis is an area of intense current research activity, propelled with significant gusto by the power of molecular genetics and proteomics. Lying immediately below the stratified epidermis, the ‘shock absorber’ dermis can be divided roughly into an upper ‘papillary’ and a lower ‘reticular’ portion, reflecting their respective composition of connective tissue components, cell number, and supply of blood vessels and nerves. Despite its greater volume, the dermis contains far fewer cells than the epidermis and instead much of its bulk consists of fibrous and amorphous extracellular matrix (ECM)33 [previously viewed wrongly as an inert ‘ground substance’] interspersed between the skin's appendages, nerves, vessels, receptors and the dermal cells. The main cell type of the dermis is the fibroblast, a heterogeneous migratory cell that makes and degrades ECM components. There is significant current interest in the factors that control the ‘differentiation’ of the dermal fibroblast,34 particularly in the context of their increased synthetic and proliferative activity during wounding healing. The dermis is home to several cell types including multi-functional cells of the immune system like macrophages and mast cells, the latter which can trigger allergic reactions by secreting bioactive mediators such as histamine.Collagen. About 90% of total dermal protein consists of the collagen macromolecule, accounting for about 75% of the skin's total dry weight. While at least 25 collagens exist, half are present in skin; consisting predominantly of collagen type I (85–90%), III (8–11%, and V (2–4%).4,33,35 Given its dominant position in skin, it is perhaps not unexpected that it can function in a diverse range of situations, from providing tissue integrity (tissue repair, migration and adhesion) to facilitating tissue morphogenesis and even platelet aggregation. The relative contribution of different collagens varies under different circumstances e.g., collagen III increases during wound healing. While most skin collagens are dermal cell-derived, endothelial cells of dermal blood vessels produce epidermal collagens.
Collagens share a 3 α-polypeptide (homotrimers or heterotrimers) chain format folded into a collagen triple helix (Fig. 9a). Every third amino acid in the chain is a glycine and the other two positions are over-represented by prolines or hyroxyprolines, with hydrogen bonding between hydroxyl groups on adjacent chains contributing to the helix's stability. The prototype collagen (collagen I) is 1000 amino acids long (300 nm) and forms a rigid rod 1.5 nm across (Fig. 9a). Collagen biosynthesis is complex, involving the synthesis of procollagen polypeptides followed by both their intracellular and extracellular processing to generate a mature and functional collagen fibre. The procollagen polypeptide is subjected to cleavage of short sequences at either or both ends and syndromes exist where such cleavage is lacking. In addition prolines are hydroxylated and lysine residues may then undergo glycosylation [addition of sugar groups]. Intra-chain and inter-chain disulfide bonds are formed before folding the macromolecule into the triple helix. Procollagen molecules are aggregated into granules for secretion purposes from the cells. Thereafter, the assembly of the molecule follows extracellular cleavage of end peptides into superstructures via covalent cross-links with other collagen types and with non-collagen molecules (Fig. 9b). A battery of enzymes is involved in collagen biosynthesis and these require a diverse range of cofactors including O2, Fe2+, ascorbate etc.
![a) Cartoon of collagen processing. The polypeptide unit is an α-chain with a glycine at every third position, which enables α-chains to wrap around each other. The resultant triple helix [pro-collagen (PC)] is secreted into the intercellular space where its ends are cleaved and thereafter organized into microfibrils (MF) and ultimately, fibrils (F). b) Collagen fibrils in human skin. Note the cross-banding with typical periodicity of approximately 64 nm. Scale bar = 65 nM.](/image/article/2006/CS/b505793k/b505793k-f9.gif) | ||
| Fig. 9 a) Cartoon of collagen processing. The polypeptide unit is an α-chain with a glycine at every third position, which enables α-chains to wrap around each other. The resultant triple helix [pro-collagen (PC)] is secreted into the intercellular space where its ends are cleaved and thereafter organized into microfibrils (MF) and ultimately, fibrils (F). b) Collagen fibrils in human skin. Note the cross-banding with typical periodicity of approximately 64 nm. Scale bar = 65 nM. | ||
Collagens are critical for epidermis adherence to the dermis35 (Fig. 8). Collagen VII forms part of the so-called anchoring fibrils that attach the basement membrane to the ECM of the upper dermis, while collagen XVII contributes to the so-called anchoring filaments that link the basal keratinocytes with the basement membrane. Basement membranes found around dermal blood vessels contain collagen VIII and XVIII. That the latter are present on the dermis side of the vascular basement membrane is of particular clinical relevance, as proteolytic cleavage of a small C-terminal fragment of this collagen, called endostatin, inhibits the formation of new blood vessel and so has tumor inhibitory properties.
Elasticity of skin. The other major ECM fibrous protein is elastin (Fig. 10), which provides the skin with its elasticity. They are stretchable by more than their full resting length. Recent biochemical analysis has clarified the composition of three different classes of elastin fibre. The first of these, termed ‘oxytalan’ fibres contains a microfibril (10–12 nm across), while the other two contain either a little (‘elaunin’ fibres) or a lot (‘elastic’ fibres) of additional amorphous components. The elasticity of the fibre derives from the amorphous portion, which contains the highly cross-linked protein called elastin. It is this tendency to form cross-links between individual elastin molecules that confers both elasticity and insolubility to these fibres. The microfibrillar part of the elastic fibre is composed principally of fibrillin-1—a large glycoprotein more than 2800 amino acids long and with Ca2+ binding repeats that aid stabilization of the molecule.
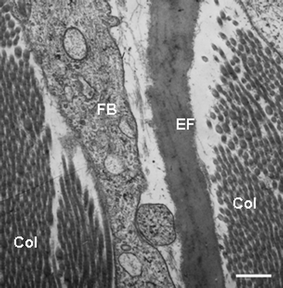 | ||
| Fig. 10 Transmission electron microscopy view of the dermis of normal human skin showing a portion of a fibroblast (FB), elastic fibre (EF) composed of the amorphous elastin with associated fibres, and obliquely sectioned collagen fibrils (Col). Scale bar = 0.25 µM | ||
Extrafibrillar matrix. The dermal material lying outside the cells, and not consisting of either collagen fibres or elastic fibres, is called the extrafibrillar matrix (Fig. 10). This amorphous and hydroscopic material is biologically very active, highly diverse and highly organized. It consists of proteoglycans, glycoproteins, water and hyaluronic acid. Proteoglycans are formed by the binding of negatively charged, water/ion-binding and sulfated/acetylated polysaccharides called glycosaminoglycans (GAGs) to a protein. Hyaluronic acid however, is an example of a GAG that is protein free. The five other physiologically relevant GAGs include chondroitin sulfate, dermatan sulfate, keratin sulfate, and heparan sulfate and heparin. The most important proteoglycan in skin is versican, produced by fibroblasts, smooth muscle cells and epithelial cells, and versican is associated with elastic fibres and hyaluronic acid to provide tautness to the skin. Another example is perlecan found in basement membranes. The Syndecans are a family of multi-functional proteoglyans involved in cell adhesion to ECM, coagulation cascades, growth factor signalling etc. Several others called ‘small leucine-rich proteoglycans’ are present in skin, each with multiple regulatory functions. The ECM also contains several other glycoproteins involved mainly in basement membrane, cell adhesion, cell migration and inter-cellular communication, e.g., laminins, fibronectin, vitronectin, matrilins, thrombospondins and tenascins etc. While on one level the ECM can be seen as complex webs of supra-structure providing a scaffold for dermal activity, the bioactivity of these macromolecules provides for exquisite regulation of numerous cellular functions.
3 Skin appendages
Given the crucial role the skin plays in body homeostasis it is not surprising that it is well-equipped with secretory [release of chemicals from cells for physiologic function] and excretory [elimination of waste products of metabolism] capability. This is conducted by sweat glands (merocrine and aprocrine), sebaceous glands, ceruminous glands (external ear canal) and mammary glands in the breast.3.1 Eccrine sweat glands
As homeotherms (warm-blooded animals) we sport 3–4 million merocrine (eccrine) glands in our skin (Fig. 2), each producing a watery perspiration that serves principally to cool us and maintain our core temperature at 37.5 °C. At maximal output the eccrine glands of an adult human can excrete as much as 3 litres per hour! This equates to a heat loss of more than 18 kcal min−1—a feat that makes human the best sweaters in the animal kingdom! Not surprising then that we insist ‘horses sweat, men perspire while women glow’! Larger and most densely packed on the palms and soles, eccrine glands have long thin ducts opening directly onto the skin surface and a proximal secretory coiled section consisting of secretory cells and contractile myoepthelial cells. The latter may be purely supportive and/or may contribute to the pulsatile nature of some sweat excretion. However, osmotic force appears enough to move the sweat.36Eccrine gland activity is regulated via neural stimulation using sympathetic nerve fibres distributed around the gland. These use the neurotransmitter acetylcholine and not the more usual adrenaline. Control of these nerves resides in so-called ‘sweat centers’ in the hypothalamus of the brain. Sweating rate is directly proportional to skin temperature and is markedly reduced at temperatures below 30 °C. While sweating can be induced by direct heating alone (39 to 46 °C), generally physiologic sweating is due to a nervous reflex that can arise from sweat centers in the brain cortex (emotional), hypothalamus (thermoregulation), and medulla (gustatory). Sweat is a clear, odour-free, colorless, slightly acidic fluid that is almost fully water (99.0–99.5%) with the remainder consisting of the electrolytes NaCl, K+ and HCO3−, and some other simple molecules (e.g., lactate, urea, ammonia, calcium, heavy metals). Raised levels of NaCl in sweat are a reliable diagnostic feature of cystic fibrosis. Recently, the full functional power of sweat became more apparent with the detection of anti-malarial peptides, proteolytic enzymes, cytokines, and even antibody (immunoglobulin A). Importantly, there are significant regional variations in the composition of sweat. The sweat gland is also known to concentrate drugs e.g., antibiotics, anti-fungal agents and chemotherapeutic drugs. Thus, the view that sweat is a waste product there only to cool us should be dispensed with. Importantly, the composition of sweat from eccrine glands can be modulated by psycho-emotional and environmental factors, and organophosphorus compounds (in insecticides and chemical warfare agents) like diisopropylfluorophosphate can induce eccrine gland sweating.
Sweat forms initially as an isotonic primary fluid in the secretory tubule that then passes through the duct for NaCl resorption, to yield a final hypotonic sweat. The steroid aldosterone is thought to regulate the reabsorption of Na+, as sweating is increased in Addison's disease where aldosterone is reduced. Several hypotheses have been proposed to explain how water and electrolytes enter sweat. An earlier Na+pump model needed positive electrical potential in eccrine gland tubules, but when a negative potential was detected instead, a more suitable model emerged akin to that in the Kidney's loop of Henle, i.e., one based on Na+, K+ and Cl− co-transportation.
3.2 Apocrine sweat/scent glands
Much less is known about the function of the larger ‘apocrine’ sweat/scent glands. While these glands secrete pheromones/hormones in lower mammals that trigger sexual and territorial responses, they are not thought to play a significant role in humans. They are found after puberty, and then only in the groin, anal region, axilla (arm-pit), areola of the breast, and beard (of adult males). Furthermore, there are significant ethnic variations in their presence and it has been widely reported (although hard data is lacking) that there is a marked reduction of these in the axillae of Koreans and Japanese.Unlike their eccrine cousins, apocrine ducts exit to the surface via the hair follicle. However, like eccrine glands, they secrete their sterile, odour-free, weakly acidic product via extrusion (exocytosis) from secretory cells. However, apocrine glands ‘sweat’ is thicker, more viscous and with a milky consistency due to its higher content of fatty acids and other compounds e.g., cholesterol, squalene, triglycerides, androgens, ammonia, sugars, ferric iron etc. Some of these are odoriferous, especially so after their decomposition on the skin surface by bacteria. Human apocrine glands are not responsive to heat, although psycho-emotional stimuli are implicated in stimulating secretory activity.
3.3 Sebaceous glands
The manner of secretion by these sebum-producing glands is unique. Their product, sebum, is released via disintegration of the sebocytes themselves. Sebaceous gland development is tightly related to the differentiation of the epidermis and hair follicle. Indeed with the exception of so-called ‘free’ sebaceous glands of mucosa (e.g., lips etc.) they are always associated with hair follicles and form so-called pilosebaceous units (Fig. 2 and 11). They are not found on palms, soles and dorsum of feet. The nature of sebum's function in humans, the ‘naked ape’ with no fur to protect, remains perplexing especially as significant evolutionary pressure must have driven the remarkable variation in the composition of sebum. The first time we humans really take notice of these glands is during puberty when they become associated with acne. Up to 800 sebaceous glands cm−2 can be found on the scalp and face; less elsewhere. While sebum production is the most obvious function of sebaceous glands, recent evidence also suggests other important roles for these glands37 in the regulation of steroidogenesis, local androgen synthesis, skin barrier function, interaction with neuropeptides, potential production of both anti- and pro-inflammatory compounds and synthesis of anti-microbial lipids (e.g. sapienate). Indeed, normal bacterial flora, fungi, Propionibacterium acnes, and even a mite called Demodex folliculorum, richly colonize sebaceous follicles.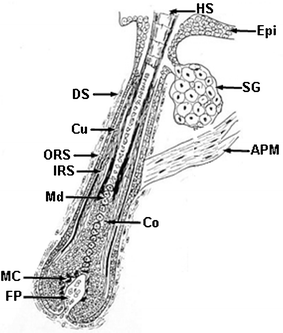 | ||
| Fig. 11 Cartoon of the longitudinal sectioned aspect of a growing human hair follicle. Key: Epi, epidermis; HS, hair shaft; SG, Sebaceous gland; APM, arrector pili muscle; DS, dermal sheath; Cu, cuticle; ORS, outer root sheath; IRS, inner root sheath; Md, medulla; Co, cortex; MC, melanocyte; FP, follicular dermal papilla. | ||
The skin contains all the enzymes needed for transformation of cholesterol to steroids. Moreover, the sebaceous glands express 5α-reductase (especially in face/scalp), needed for the intracellular conversion of testosterone to the more potent 5α-dihydrotestosterone. Moreover, these glands are part of the skin neuroendocrine system as they produce and release corticotrophin-releasing hormone (in response to stress). The human sebaceous gland is both glycolytic and glutaminolytic; the sugar glucose is converted to lactate, and glutamine is converted to glutamate, alanine, serine, glycine, threonine, lactate and ammonia. Recently, it has been shown that exogenous glutamine is required for sebocyte division and lipogenesis, though it can be replaced by spermidine.
Sebum is a yellowish viscous fluid containing triglycerides, free sterols and sterol esters, squalenes, wax and free fatty acids. Some of the latter may be converted from surface triglycerides by bacterial hydrolases, although sebaceous glands can also synthesize considerable amounts of free fatty acids. Early work on the hydrolysis of human sebum revealed how unusual sebum is. For example, approximately half of the fatty acids in sebum are monosaturated with some unusually positioned double bonds (e.g. Δ6 unsaturation) unique to humans. Moreover, alcohol moieties of the wax esters also exhibit unusual chain types similar to those of the fatty acids. Another unusual feature is its high squalene content. The composition of sebaceous gland lipids (including the relative proportions of different types of branched-chain fatty acids) appears to be under both genetic and hormonal control and while significant inter-individual and inter-ethnic differences in sebum production exist, a rate of 0.3 mg sebum per 10 cm3 per hour is normal.38
3.4 The hair follicle
Hair follicles (and mammary glands) mark us out as mammals. Thus, we can be forgiven for assuming these skin appendages had/have important functions. Further kudos to the hair follicle is that it is our body's only permanently regenerating organ, as it transits through life-long periods of growth (anagen), regression (catagen) and relative quiescence (telogen). Five million hair follicles reside in our skin (Fig. 2 and 11), though only a paltry 2% are on our heads (even less on mine!). The most ‘alabaster’-skinned have just as many of these mini-organs as the ‘hairiest’ of us—with hair visibility depending on hair size and distribution rather than number.While it is clear that hair serves a critical role of thermoregulation in furry animals, its role in humans appears to be non-essential. Still, there is much potency in the hair ‘signal’ for humans, and significant psychological trauma associated with hair ‘problems’, either too much, too little or of the ‘wrong’ type, and color etc. For an up-to-date discussion of hair follicle anatomy and physiology, the interested reader is directed to a recent text on hair.39 The hair follicle encapsulates all the important physiologic processes found in the human body namely; controlled cell growth/death, interactions between cells of different histologic type, cell differentiation and migration, hormone responsitivity etc. An important consideration for those interested in the chemistry of skin is that hair fibre growth occurs in a highly time-resolved manner and so ‘locks in’ a snap-shot of the individual's physiology and chemistry at the time of hair fibre's formation. The successful maintenance of human hair follicle growth in vitro has permitted analysis of the biochemistry of hair growth.40 Using this system, glucose (and less so glutamine) was shown to be the principal energy source.
This hair follicle mini-organ deserves our further admiration for its ability to intersect with the body's systemic regulatory networks. Remarkably, the hair follicle can respond to most hormones known to biomedicine. Even more surprising is the hair follicle's capacity to produce for itself a wide range of hormones e.g., sex steroid hormones, proopiomelanocortin peptides, corticotrophin-releasing hormone, prolactin etc. Further, neuro-peptides, -transmitters and -hormones are implicated in mediating hair follicle events particularly those related to stress. The observation that men castrated before puberty do not go bald nor grow beards, but did so after treatment with so-called male hormone testosterone, indicated a role of androgens in hair growth. Crucially, hair follicles in different regions of the body respond differently to different androgens, and inhibition of testosterone-converting enzyme, Type II 5α-reductase, by the drug finasteride, induces hair re-growth in some balding men.
Another big surprise to cutaneous scientists was the hair follicle's unique immunological status. Unlike the rest of the skin, the lower portion of growing hair follicles is ‘immuno-silent’ due to its lacking tissue histocompatibility antigens. The hair follicle may enjoy ‘immune privilege’ to prevent the inappropriate recognition of proteins that may result in the attack of cells expressing these protein antigens. While hair canals contain a resident microflora of bacteria including P. acnes, Staphylococcus aureus, S. epidermidis, Demodex follicularum, Malassezia speciesetc., the hair follicle appears to have a very effective anti-infection capacity, as evidenced by the rarity of folliculitis in human scalp despite its 100,000 or so individual hair follicles.
One of the long enduring enigmas of dermatologic research is the nature of the ‘clock’ controlling hair cycling. Many hypotheses have attempted to explain this, though none can yet fully explain all aspects of the hair growth cycle. The “bulge-activation” hypothesis is currently favored.41 This suggests that at the end of resting phase (telogen) a unique group of epithelial cells (‘stem cells’) are activated in the upper hair follicle ‘bulge’. This stimulation is followed by stem cell proliferation and their progeny go on to reform the lower ‘temporary’ part of the hair follicle in anagen. Unlike the stem cell, proliferating ‘transient amplifying’ cells have limited mitotic [dividing] potential before undergoing differentiation.
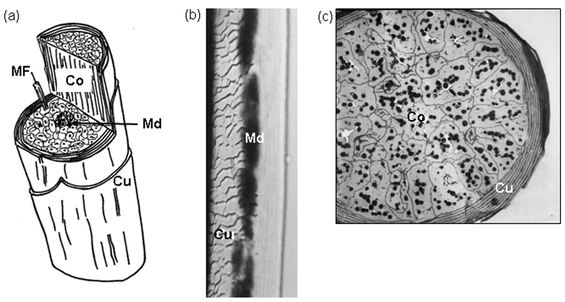 | ||
| Fig. 12 a) Cartoon of the hair shaft with cut-through to reveal inner aspects. Co, cortex; Cu, cuticle; Md, medulla. A macrofibril (300 nm) of single cortical cell is revealed with a constituent microfibril (MF; 7–10 nm). b) Whole mount of a scalp hair shaft with focal plane on cuticle (Cu, left) and medulla (Md; middle). c) Transverse section of a fine hair shaft revealing a multilayered cuticle (Cu) encompassing a tightly packed cortex (Co) containing many dark melanin granules. Scale bars b = 14 µM, c = 4.5 µM. | ||
The highly complex field of hair or ‘hard’ α-keratins (i.e., hair, nail, claw etc.) has benefited much from recent advances in molecular biology. The ‘hard’ hair keratins (7–8 nm across) can be distinguished from epidermal ‘soft’ keratins by their lack of extended glycine runs. Instead ‘hard’ hair keratins contain many cysteine residues (particularly at the N- and C-terminal domains) that enable them to form extensive disulfide bond cross-linking with other cysteine-rich proteins. Moreover, the dynamics of assembly of keratin intermediate filaments in epidermis and hair fibres is very different. Whereas these filaments disassemble during epidermal keratinocyte division, they are the product of non-viable keratin-producing cortical keratinocytes in the hair follicle. In order to form a rigid and resistant hair shaft, abundant cysteine residues of the hair keratins need to be extensively cross-linked by disulfide bonds. Keratin-associated proteins (KAPs) facilitate such cross-linking, and various analytical tools (e.g., solubilization, chromatography, electrophoresis, amino acid sequencing etc.) have revealed an increasingly complex group of proteins. At least 23 KAP families are now known, although research from the last couple of years promises to reveal even greater complexity, with large clusters of novel human KAP genes now located.39
Lipids on the hair fibre surface (e.g. cuticle) provide a hydrophobic interface protecting the hair cortex from a hostile wet/dry environment. It was not until the mid 1980s when a lipidic layer on the surface of hair fibres—termed the F-layer—was discovered, and this was found to contain a large amount (58% of total) of a methyl-branched 21-carbon fatty acid. The 21-carbon saturated fatty acid (exclusive to the hair/wool cuticle) is present in exceptionally high amounts, and has been identified as 18-methyl-eicosanoic acid by mass spectroscopy. The main function of the branched methyl-eicosanoic acid is currently unknown, but it may be involved with increasing the degree of hydrophobicity over straight-chain fatty acids and/or altering the frictional quality of the fibre. In humans the major fatty acids in hair fibre lipids include 16∶0 (17%), 18∶0 (10%), 18∶1 (5%) 21∶0 (48%). It is of some considerable interest that hair cuticle lipids are highly conserved; in marked contrast to the high inter-species variability in sebaceous gland lipids (see section 3.3). Evidence that lipids are surface-bound is inferred from data showing that increasing hair fibre diameter is associated with a decrease in total bound fatty acid. Thus, it is now well appreciated that hair fibres have a 3–30 nm coating of long-chain fatty acids bonded covalently to the protein membrane of the epicuticle. Recent technologic advances, e.g., in atomic force microscopy, have allowed exceptionally high power views of the hair fibre surface, and of how this is altered by changes in temperature, hydration, pH, lipid layer removers, topically-applied cosmetic products etc. Small amounts of cholesterol sulfate, cholesterol, and fatty alcohol are also associated with the F-layer, though the nature of bonding (e.g.via thioester linkages) is unclear.
Human hair is commonly grouped into just three main sub-types: Caucasian, Mongoloid Asian and African. Differences between these groups are usually determined with respect to a range of parameters including: hair fibre diameter and its cross-sectional form, overall fibre shape, mechanical properties (see above), combability, shape, chemical make-up and moisture level.42 For many of these parameters Caucasian hair falls intermediate to the Asian and African extremes. Recently, a systematic examination of the protein structure of hairs from Asian, Caucasian and African individuals revealed no differences by X-ray analysis in the structure of the hair keratin. Asian and Caucasian hair fibres are more cylindric than those of Africans, and it has been shown that ‘breaking stress’ and ‘breaking extension’ values are lower in African hair fibres than in Caucasians or Asians. Meanwhile, despite differences in diameters of Asian and Caucasian hairs, both exhibit similar behaviours during stress. Despite these gross ethnic differences in the form of hair fibres, the chemistry of the hair keratin is remarkably similar throughout all humans. The compositional or structural features of hair protein in these ethnicities thus do not provide immediate explanations for their behavioural differences.
3.5 The nail
The nail is the least well studied of the skin appendages. Apparently derived from the earlier claw, this hard, keratinized, appendage protects the distal end of the fingers and toes. When we speak of the nail, we are commonly referring just to the nail plate that overlays the highly vascularized nail bed and so appears pinkish. The cells that go to make up the nail plate are similar to those generating the corneocytes of the upper epidermis and the trichocytes that make the hair shaft. So-called onychocytes in the nail are also filled with keratin filaments. Low-sulfur keratins form 10 nm α-helical filaments running parallel to the nail surface and these are embedded in a non-keratin matrix material of high sulfur, high glycine, high tyrosine proteins. Up to 90% of nail keratins are of the hard ‘hair’ keratin type; the remainder are soft ‘epidermal’ keratins. Also present in nail are water, lipids, and trace elements including iron, zinc, and calcium. Overall, the nail contains much less lipid and water than the epidermis and become brittle at less than 7% water and soft at more than 30%. The nail’s low lipid content contributes to their 1000 times greater water permeability than the epidermis.4 Cutaneous neuro-endocrinology
The last 10 years has seen much excitement regarding the discovery of the huge capacity of skin and its appendages to act as a neuroendocrine organ in the periphery, and doing so in a manner largely independent of control from the body's traditional and central stress system.1,2 The skin occupies a strategic location between the external and internal environments and so it could be expected to contribute to preserving body homeostasis [maintenance of constancy of body's internal environment]. The cutaneous neuroendocrine system provides an over-arching capacity for stress sensing. Particular importance lies in its having an equivalent of the central hypothalamic–pituitary–adrenal axis. While this ‘stress system’ in skin may not be similarly organized structurally (i.e. different tissues for different functionality), it is still is organized with functionalities residing in the same tissue and sometimes even within the same cell type. Its principal constituent activities include: the production of corticotrophin-releasing hormone (and downstream proopiomelanocortin peptides, including endorphins); steroido-genesis [synthesis, metabolism and targeting of androgens and estrogens]; secosteroido-genesis [steroids after fission of one or more ring structures with concomitant addition of a hydrogen atom at each terminal group], e.g. UVB-induced splitting of 7-dehydrocholesterol to produce Vit D3; and a serotinergic/melatoninergic system (e.g. serotonin: pro-inflammatory, pro-edema, vasodilatory, pro-pruritogenic and melatonin: hair growth control, pigmentation etc.). Furthermore, a hypothalamic–pituitary–thyroid equivalent also appears to be expressed in the skin, most dramatically indicated by the expression of functional receptors for thyroid stimulating hormone.15 Pigmentation of skin and hair follicle
Of all our phenotypic traits skin and hair color communicate more immediate information to the observer than any other. Humans display a rich and varied palette of surface color that not only highlights striking superficial variations between human sub-groups, but also underscores how we differ phenotypically from other mammalian species. Skin color has traditionally been defined by the Fitzpatrick classification I–VI; where Type I – Always burns, never tans; II – Burns easily, tans minimally; III – Burns moderately, tans gradually to light brown; IV – Burns minimally, always tans well to moderately brown; V – Rarely burns, tans profusely to dark; VI – Never burns, deeply pigmented.However, recent evidence suggests that the out-bred nature of humans does not make them fall so neatly into these categories. While, skin color and ethnicity are associated, ethnicity alone may not predict susceptibility to UV-related skin damage. Skin color depends principally on relative amounts of eumelanin (brown/black) and pheomelanin (red/yellow), and less to hemoglobin and carotenoids.
Meanwhile, the colors of our scalp hair can range from vivid reds and sun-bleached blondes to sober browns, raven blacks and with age to steel greys and snow whites. Despite such variation, all skin and hair color is derived from the pigment melanin, synthesized via a phylogenetically ancient biochemical process termed melanogenesis.43 Synthesis occurs within melanosomes—specialized organelles unique to highly dendritic, neural crest-derived, cells called melanocytes (Fig. 13 and 14b). While follicular melanocytes are derived from epidermal melanocytes during hair follicle development, these pigment cell sub-populations diverge in many important ways. For example, melanin degrades almost completely in the differentiating layers of the epidermis, whereas melanin granules (especially eumelanin) transferred into hair cortical keratinocytes remains minimally digested (Fig. 14 a and d). In addition, while activity of the hair bulb melanocyte occurs only during the anagen phase of the hair growth cycle, epidermal melanogenesis is continuous.
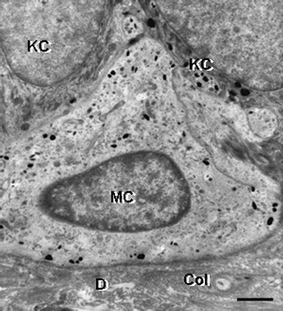 | ||
| Fig. 13 Transmission electron microscopy view of a melanocyte (MC) in the basal layer of the epidermis of human skin. Note the presence of fine melanin granules in the clear cytoplasm of a melanocyte, which is in close apposition to two keratinocytes (KC). One KC contains multiple transferred melanin granules. D, dermis; Col, collagen. Scale bar = 1.3 µM. | ||
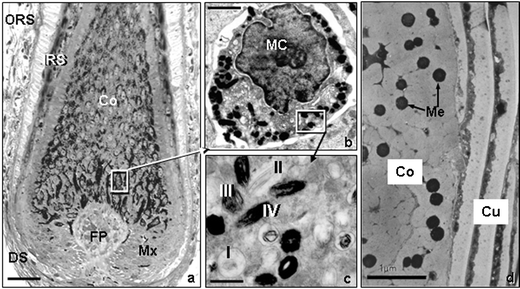 | ||
| Fig. 14 a) High-resolution light microscopy view of the lower part of a growing scalp hair follicle. Note the location of the melanin in cells located around the follicular dermal papilla (FP) and its subsequent transfer to the cortical (Co) cells above, which produces the hair shaft. b) Transmission electron microscopy view of a single hair bulb melanocyte (MC). c) Transmission electron microscopy view of the melanin-producing granules called melanosomes. Note the four stages of maturation of these granules. d) Transmission electron microscopy view of a transversly-cut hair shaft showing melanin granules (Mg) located between keratin macrofibrils in the hair cortex (Co), but none are found in the hair cuticle (Cu). Scale bar a; 28 µM b; 3.2 µM c; 1 µM d; 1 µM. | ||
The pigmentation of melanocytes in skin and hair follicles is affected by numerous extrinsic and intrinsic factors including; body distribution, ethnicity/gender differences, variable hormone-responsiveness, genetic defects, hair-cycle dependent changes, age, UVR, climate/season, toxin, pollutants, chemical exposure and infestations. In contrast to its direct regulatory role in epidermal pigmentation, UVR does not penetrate to the melanogenic cells of the anagen hair bulb (Fig. 14a).
The process of melanogenesis can be divided into a) the formation of the melanosome in which melanogenesis occurs (Fig. 14c) and b) the biochemical pathway that converts L-tyrosine into melanin (Fig. 15). Both processes are under complex genetic control. At least three melanogenic enzymes are involved including the rate-limiting tyrosinase (with both tyrosine hydroxylase and Dopa oxidase activities) and tyrosinase-related proteins [TRP-1 and DCT (TRP-2)], which are also important for maintaining the stability of tyrosinase at the melanosomal membrane. Eumelanogenesis (brown-black melanin production) is critically dependent on the velocity of the tyrosinase reaction, although this is also stimulated by TRP-1 and DCT. For pheomelanogenesis (red/yellow melanin production), cysteinyl-Dopa is oxidized in multiple complex steps that may involve tyrosinase-dependent/-independent reactions as well as glutathione reductase and peroxidase activities, in order to form pheomelanin.
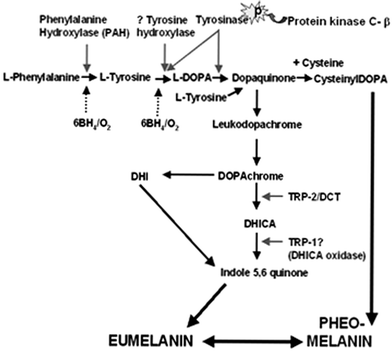 | ||
| Fig. 15 The biosynthesis of melanins is based on the conversion of the amino acid L-tyrosine (additionally via L-phenylalanine) into a complex and heterogeneous group of compounds. This reaction can be broadly divided into the following steps: 1) hydroxylation of L-phenylalanine/L-tyrosine to L-dihydroxy-phenylalanine (L-Dopa), 2) The dehydrogenation/oxidation of L-Dopa to dopaquinone—the precursor for both eu/pheomelanins and the limiting step in melanogenesis. 3) The dehydrogenation of DHI (dihydroxyindole) to yield melanin pigment. Eumelanogenesis and pheomelanogenesis both require the oxidation of Dopa to dopaquinone. Thereafter, the conversion of dopaquinone to leuko-dopachrome signals eumelanin production, while the addition of cysteine to dopaquinone to yield cysteinyldopa occurs in pheomelanin production. For eumelanogenesis (brown/black), L-Dopa needs to be oxidized by tyrosinase to L-dopaquinone and again by tyrosinase from DHI to indole-5,6-quinone. 6BH4; 6-tetrahydrobiopterin ; L-DOPA, L-3,4-dihydroxyphenylalanine; DHI, dihydroxyindole; DCT, dopachrome tautomerase; TRP-1/2, tyrosinase related protein-1/2; DHICA, dihydroxyindole carboxylic acid; | ||
The exact details of the melanogenesis are still a matter of intense research, and beyond the scope of this review.43 Recently, there has been some re-evaluation of the enzymology of the classical Raper–Mason pathway for melanogenesis.43 It appears that the initial hydroxylation of tyrosine to Dopa may also occur via a second enzyme tyrosine hydroxylase (isoform I in humans) (Fig. 15), which may be localized in the melanin granule in humans. Moreover, the involvement of the tetrahydropteridines (as essential co-factor of aromatic hydroxylases) in regulating phenylalanine hydroxylase (converting phenylalanine to tyrosine) and tyrosine hydroxylase (converting tyrosine to Dopa) may be important for early stages of melanogenesis. Furthermore, it has been proposed that the enzyme protein kinase Cβ may also participate by modulating the activity of tyrosinase during the oxidation of Dopa portion of the pathway43 (Fig. 15).
Melanins are polymorphous and multifunctional biopolymers that include the cutaneous melanins; eumelanin, pheomelanin and mixed melanins (containing both eumelanin and pheomelanins), and neuromelanin. Despite the broad association of eumelanin with brown/black hair and pheomelanins with red/blonde hair, relatively minor differences in melanin content can have significant effects on visible hair color. Moreover, both eu- and pheomelanin can be produced within the same melanocyte. Melanin in hair is tightly bound to the keratin and its isolation requires degradation of keratin by strong chemicals (e.g. 1 M NaOH and conc. HCL). Melanin pigments have a common arrangement of repeating units linked by carbon–carbon bonds, although they differ from each other with regard to their chemical, structural and physical properties. Eumelanins are polymorphous nitrogenous biopolymers (mostly co-polymers of dihyroxyindole and dihyroxyindole carboxylic acid) (Fig. 15). They are insoluble in most solvents and are tightly associated with proteins through covalent bonds. Eumelanins behave like polyanions and are capable of binding reversibly to cations, anions and polyamines—reactions facilitated by their high carboxyl group content. The semi-quinone units of eumelanin provide it with unique stable paramagnetic states. Thus, the electron paramagnetic resonance (EPR) spectrum of eumelanin reflects its slightly asymmetric singlet that generates a free radical signal. The semiquinone units of eumelanin are also responsible for its redox status, with both reducing and oxidizing capabilities towards oxygen radicals and other chemical redox systems. Unlike eumelanin, alkali-soluble pheomelanin has a backbone of benzothiazine units with high variability in nitrogen and sulfur content (C/N and C/S ratios).
Melanins are tightly bound to protein (i.e. detergents can't dissociate proteins from the melanin completely). Removal of SDS from melanoprotein samples results in the rebinding of different proteins to the melanin according to their own binding km and specific stoichiometries. Thus, we can infer some information regarding the nature of protein binding (i.e. likely ionic) to the anionic melanin polymer.44 Like eumelanin, pheomelanin can act as a binding agent for drugs and chemicals and contains semiquinones with their associated paramagnetic properties. However, pheomelanins have additional semiquinoneimine centers, resulting in different EPR spectra thereby allowing identification of melanin type and quantification. Pheomelanins are photo-labile with photolysis products including superoxide, hydroxyl radicals and hydrogen peroxide.
6 The skin immune system
It is only appropriate that the immune function should be strongly represented in the organ that is most directly responsible for separating physically the self from the non-self. However, the skin not only provides immune protection for itself, but also helps protect the whole body. More stimulation of the immune system is likely to occur at this biological interface (between you and your external environment) than any other area of your body. Biological aggressors like bacteria, viruses, mould, yeast, fungus and chemical insults that threaten our health can gain entry to our bodies via the skin and its numerous ports of entry (e.g. hair follicle canals, sweat gland pores etc.) However, unless the skin itself is damaged (e.g. wounds, abrasions or disease) or the host is compromised (e.g. immuno-suppressed), most of these threats are repelled by our skin.Immunodermatology, the study of the skin immune system (SIS), has grown enormously over the last couple of decades and we have made great strides in the dissection of the skin immunological networks involved in both physiologic and pathologic circumstances.45 The make up of the SIS can be most simply described in terms of its components, i.e. cellular and humoral components or alternately whether we are looking at its innate (pre-existing) or adaptive (subsequent to prior exposure to an immune response generating stimulus) immunity. In the last few years it has become clear that practically all cell types residing in and transiting through skin can exhibit immune functionality. The better characterised cellular components include keratinocytes, lymphocytes (various subpopulations in skin), Langerhans cells (and other skin dendritic cells), monocytes and macrophages, endothelial cells of blood and lymphatic vessels, mast cells (containing a battery of mediators of both immune and neuroendocrine responses), neutrophils, eosinophils, and basophils. Innate components include free radicals, anti-microbial peptides including defensins and cathelicidins, cytokines, chemokines, neuropeptides, adhesion molecules and a wide range of pro- and anti-inflammatory mediators. Immunoglobulins or antibodies are additional potent proteins that are secreted from activated B-lympocytes and can neutralize threats to the body. Given the surface location of skin, it is not surprising that the physiology and pathology of skin can be affected by ultraviolet sunlight and so the sub-field of photo-immunology has yielded several important findings. Prominent among these is the general immuno-suppressive effect of sunlight with its associate killing of Langerhans cells or altering their function. Our increasing longevity will require us to depend even more on the skin immune system immuno-surveillance function to prevent and limit tumour growth etc.
There are also may congenital and acquired disorders with associated skin manifestations, as well as immune or autoimmune related disorders. Examples, of the latter include so-called immunobullous or blistering diseases, Lichen planus and graft-versus-host disease, Lupus erythematosus, psoriasis etc. Moreover, how the skin handles xenobiotics also involves immunological mechanisms, and the degree to which individuals may exhibit an allergic or atopic tendency will determine whether they will develop atopic dermatitis, allergic contact dermatitis etc. The last decade or so has witnessed the development of new immuno-modulatory drugs (both biologics and chemicals) for manipulating skin immune response and so managing treatment of skin disease.
The hair follicle has its own unique immune system (see section 3.4) principally characterised by its so-called immune privileged status, where its lower regions are immuno-silent during parts of the hair growth cycle. This appears to be an adaptation to protect this critical mini-organ during its hair cycle-dependent dramatic tissue remodelling events.46
7 Nutrition and the skin
Skin and hair disorders have long been associated with nutritional deficiencies.47 More recently we have become particularly interested in the relationship between food and health both via its intake and its absorption/metabolism. Dermatologic conditions linked with nutrition can range from gross malnutrition effects and more subtle protein/protein-calorie deficiencies (e.g., kwashiorkor/marasmus) to specific vitamin/trace element deficiencies and metabolic disorders. Deficiency of essential fatty acids [fatty acids derived from diet] e.g., linoleic and linolenic acids, can cause the skin to become dry, scaly, leathery, reddened and with increased TEWL. Hair is commonly lost from the scalp (i.e. alopecia). Essential fatty acids make up to 30% of the skin’s supply of fatty acids. Another common category involves vitamin deficiencies. Vitamin A (in animal fats, milk and a little in green leafy vegetables) is critical for epidermal function and is needed to prevent hyper-keratinization. Vitamin K (in meat and green leafy vegetables etc.) is important in the synthesis of coagulation factors. Deficiency of vitamin K can lead to sub-dermal bleeding. Water-soluble vitamin C (in fresh fruit and vegetables) plays an important role in the synthesis of collagen and ECM. A deficiency of vitamin C can result in scurvy with associated skin symptoms of hair follicle hyperkeratosis, formation of corkscrew hairs, and bleeding of skin and gums. Cutaneous defects are also associated with lack of vitamin B complex with skin manifestation depending on the type of member affected. Common examples include skin ulceration and conjunctivitis (vitamin B2); pellagra [hyperpigmentation with skin sloughing] (vitamin B3); pernicious anemia with associated poliosis [type ofhair depigmentation], vitiligo, [type ofskin depigmentation], alopecia areata [type ofpatchy or universal hairloss] (vitamin B12); alopecia, eczema, conjunctivitis (Biotin or vitamin H).Inadequate supply of trace element/minerals, most commonly involving copper, zinc and selenium, is another category of nutritional deficiency with potential cutaneous manifestations. Copper deficiency is rare; skin problems result from metabolic disorders of copper. Several important enzymes in the skin use copper (e.g. tyrosinase for pigmentation). Menke's syndrome presents with kinky, twisted hair (so-called pili torti) with periodic narrowing of the hair shaft and is due to a mutation in the gene encoding for a Cu2+ transporting enzyme. Zinc is the most important mineral for human health and is ingested via our diet (nuts, grains, green leafy vegetables etc.). Unlike for copper, a deficiency in zinc can be due either to nutrition or to an inherited disorder. Dermatitis [erosions of the skin], alopecia, nail defects are associated with a lack of zinc. The wool literature contains several reports suggesting that correction of deficiencies in copper and zinc can improve wool fibre growth. Finally, a deficiency in selenium will disturb our protection against oxidative damage, as this mineral is a component of the enzyme glutathione peroxidase. Associated skin signs include hypopigmentation of the skin and hair and whitening of the nails.
8 Concluding remarks
I hope I have been able to give you a general flavour of our current thinking of the biochemistry of skin. We are now entering a terrifically exciting period of cutaneous research with a well-honed armamentarium of new skills based on the genomic and proteomic technologies. The full interpretation of much of these data however, will require focusing through the prism of biochemistry. How important is biochemistry? Well, this reminds me of a conversation I had as a university freshman. When asked by one of my new peers which subject I was studying for I replied ‘Biology.’ ‘Ah my dear old pal’, (we'd just met), ‘you know what? Chemistry is the Super-Biology.’ ‘Fine.’ I replied, which alas only got him going. He continued, ‘Physics is the Super-Chemistry and Astrophysics the Super-Physics.’ ‘Fine, Fine, Fine.’ I interrupted, unmoved. But then with a Mona Lisa-like smile he concluded, ‘Ah and guess what? Theology, of course, is the Super-Astrophysics…’References
- A. Slominski, Neuroendocrine system of the skin, Dermatology, 2005, 211 DOI:10.1159/000087012.
- C. M. Chuong, B. J. Nickoloff, P. M. Elias, L. A. Goldsmith, E. Macher, P. A. Maderson, J. P. Sundberg, H. Tagami, P. M. Plonka, K Thestrup-Pederson, B. A. Bernard, J. M. Schroder, P. Dotto, C. M. Chang, M. L. Williams, K. R. Feingold, L. E. King, A. M. Kligman, J. L. Rees and E. Christophers, What is the ‘true’ function of skin?, Exp. Dermatol., 2002, 11(2), 159–87 CrossRef CAS.
- M. Haeney, The immunological background to transplantation, J. Antimicrob. Chemother., 1995, 36(Suppl B), 1–9 CAS.
- L. A. Goldsmith, Physiology, biochemistry, and molecular biology of the skin, Oxford University Press, New York, 1991 Search PubMed.
- R. K. Freinkel and D. T. Woodley, The biology of skin, Parthenon Publishing, New York, 2001 Search PubMed.
- L. A. Goldsmith, My organ is bigger than your organ, Arch. Dermatol., 1990, 126, 301–2 CAS.
- I. Moll, M. Roessler, J. M. Brandner, A. C. Eispert, P. Houdek and R. Moll, Human Merkel cells–aspects of cell biology, distribution and functions, Eur. J. Cell Biol., 2005, 84(2–3), 259–71 Search PubMed.
- G. S. Sidhu, P. Chandra and N. D. Cassai, Merkel cells, normal and neoplastic: an update, Ultrastruct. Pathol., 2005, 29(3–4), 287–94 CrossRef.
- E. B. Lane and W. H. McLean, Keratins and skin disorders, J. Pathol., 2004, 204(4), 355–66 CrossRef CAS.
- L. Langbein and J. Schweizer, Keratins of the human hair follicle, Int. Rev. Cytol., 2005, 243, 1–78 Search PubMed.
- R. Moll, Molecular diversity of cytokeratins: significance for cell and tumor differentiation, Acta Histochem. Suppl., 1991, 41, 117–27 Search PubMed.
- P. M. Elias and G. K. Menon, Structural and lipid biochemical correlates of the epidermal permeability barrier, Adv. Lipid Res., 1991, 24, 1–26 Search PubMed.
- D. A. Weigand, C. Haygood and J. R. Gaylor, Cell layers and density of Negro and Caucasian stratum corneum, J. Invest. Dermatol., 1974, 62(6), 563–568 CrossRef CAS.
- M. Griffin, R. Casadio and C. M. Bergamini, Transglutaminases: nature's biological glues, Biochem. J., 2002, 1;368(Pt 2), 377–96 CrossRef.
- P. L. Zeeuwen, Epidermal differentiation: the role of proteases and their inhibitors, Eur. J. Cell Biol., 2004, 83(11–12), 761–73 Search PubMed.
- D. J. Tobin, K. Foitzik, T. Reinheckel, L. Mecklenburg, V. A. Botchkarev, C. Peters and R. Paus, The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling, Am. J. Pathol., 2002, 160(5), 1807–21 Search PubMed.
- S. Hasse, S. Kothari, H. Rokos, S. Kauser, N. Y. Schurer and K. U. Schallreuter, In vivo and in vitro evidence for autocrine DCoH/HNF-1alpha transcription of albumin in the human epidermis, Exp. Dermatol., 2005, 14(3), 182–7 CrossRef CAS.
- L. Coderch, O. Lopez, A. de la Maza and J. L. Parra, Ceramides and skin function, Am. J. Clin. Dermatol., 2003, 4(2), 107–29 Search PubMed.
- J. A. Bouwstra, J. Thewalt, G. S. Gooris and N. Kitson, A model membrane approach to the epidermal permeability barrier: an X-ray diffraction study, Biochemistry, 1997, 36(25), 7717–25 CrossRef CAS.
- V. A. Ziboh, Y. Cho, I. Mani and S. Xi, Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin, Arch. Pharm. Res., 2002, 25, 747–58 Search PubMed.
- L. Du, S. M. Hoffman and D. S. Keeney, Epidermal CYP2 family cytochromes P450, Toxicol. Appl. Pharmacol., 2004, 195, 278–87 CrossRef CAS.
- J. E. Storm, S. W. Collier, R. F. Stewart and R. L. Bronaugh, Metabolism of xenobiotics during percutaneous penetration: role of absorption rate and cutaneous enzyme activity, Fundam. Appl. Toxicol., 1990, 15(1), 132–41 CrossRef CAS.
- H. I. Swanson, Cytochrome P450 expression in human keratinocytes: an aryl hydrocarbon receptor perspective, Chem. Biol. Interact., 2004, 149(2–3), 69–79 CrossRef CAS.
- N. Ahmad and H. Mukhtar, Cytochrome p450: a target for drug development for skin diseases, J. Invest. Dermatol., 2004, 123(3), 417–25 CrossRef CAS.
- R. L. Eckert, T. Efimova, S. R. Dashti, S. Balasubramanian, A. Deucher, J. F. Crish, M. Sturniolo and F. Bone, Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase, J. Invest. Dermatol. Symp. Proc., 2002, 7(1), 36–40 Search PubMed.
- L. Alonso and E. Fuchs, Stem cells of the skin epithelium, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 11830–5 CrossRef CAS.
- J. R. Bickenbach and K. L. Grinnell, Epidermal stem cells: interactions in developmental environments, Differentiation, 2004, 72(8), 371–80 CrossRef.
- K. Y. Sarin, P. Cheung, D. Gilison, E. Lee, R. I. Tennen, E. Wang, M. K. Artandi, A. E. Oro and S. E. Artandi, Conditional telomerase induction causes proliferation of hair follicle stem cells, Nature, 2005, 436(7053), 1048–52 CrossRef CAS.
- S. Gingras, C. Turgeon, N. Brochu, P. Soucy, F. Labrie and J. Simard, Characterization and modulation of sex steroid metabolizing activity in normal human keratinocytes in primary culture and HaCaT cells, J. Steroid Biochem. Mol. Biol., 2003, 87(2–3), 167–79 CrossRef CAS.
- A. A. Dlugosz and S. H. Yuspa, Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C, J. Cell Biol., 120, 217–225 Search PubMed.
- M. Manabe and W. M. O'Guin, Keratohyalin, trichohyalin and keratohyalin-trichohyalin hybrid granules: an overview, J. Dermatol., 1992, 19(11), 749–55 Search PubMed.
- L. R. Lund, J. Romer, T. H. Bugge, B. S. Nielsen, T. L. Frandsen, J. L. Degen, R. W. Stephens and K. Dano, Functional overlap between two classes of matrix-degrading proteases in wound healing, EMBO J., 1999 Sept. 1, 18(17), 4645–56 Search PubMed.
- L. Brucker-Tuderman, Biology of the extracellular matrix, in Dermatology, ed. J. L. Bolognia, J. L. Jorizzo and R. P. Rapini, Mosby, Edinburgh, 2003 Search PubMed.
- H. Y. Chang, J. T. Chi, S. Dudoit, C. Bondre, M. van de Rijn, D. Botstein and P. O. Brown, Diversity, topographic differentiation, and positional memory in human fibroblasts, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(20), 12877–82 CrossRef CAS.
- J. Uitto, Connective tissue biochemistry of the aging dermis. Age-associated alterations in collagen and elastin, Clin. Geriatr. Med., 1989, 5(1), 127–47 Search PubMed.
- K. Saga, Structure and function of human sweat glands studied with histochemistry and cytochemistry, Prog. Histochem. Cytochem., 2002, 37(4), 323–86 Search PubMed.
- C. C. Zouboulis, Acne and sebaceous gland function, Clin. Dermatol., 2004, 22, 360–366 CrossRef.
- G. Plewig and A. M. Kligman, in Acne and rosacea. 3rd Edn, Berlin, Springer, 2000 Search PubMed.
- D. J. Tobin, in Hair in Toxicology – an important biomonitor, ed. D. J. Tobin, Royal Society of Chemistry, Cambridge, 2005 Search PubMed.
- M. P. Philpott and T. Kealey, Metabolic studies on isolated hair follicles: hair follicles engage in aerobic glycolysis and do not demonstrate the glucose fatty acid cycle, J. Invest. Dermatol., 1991, 96(6), 875–9 CrossRef CAS.
- R. J. Morris, Y. Liu, L. Marles, Z. Yang, C. Trempus, S. Li, J. S. Lin, J. A. Sawicki and G. Cotsarelis, Capturing and profiling adult hair follicle stem cells, Nat. Biotechnol., 2004, 22(4), 411–7 CrossRef CAS.
- C. R. Robbins, Chemical and Physical behavior of human hair, Springer, New York, 2002 Search PubMed.
- A. Slominski, D. J. Tobin, S. Shibahara and J. Wortsman, Melanin pigmentation in mammalian skin and its hormonal regulation, Physiol. Rev., 2004, 84(4), 1155–228 Search PubMed.
- S. Sharma, S. Wagh and R. Govindarajan, Melanosomal proteins–role in melanin polymerization, Pigment Cell Res., 2002, 15(2), 127–33 CrossRef CAS.
- J. D. Bos, in Skin Immune System: Cutaneous Immunology and Clinical Immunodermatology, 3rd Edn., ed. Jan D. Bos, CRC Press, Boca Raton, 2004 Search PubMed.
- T. Christoph, S. Muller-Rover, H. Audring, D. J. Tobin, B. Hermes, G. Cotsarelis, R. Ruckert and R. Paus, The human hair follicle immune system: cellular composition and immune privilege, Br. J. Dermatol, .2000, 142(5), 862–73 Search PubMed.
- R. Ruiz-Maldonado and G. Becerril-Chihu, Skin manifestations of malnutrition, in Text book of pediatric dermatology, ed. J. Harper, A. Oranje and N. Prose, Blackwell, London, 2000, pp 499–506 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
