Fluorescence quenching immunoassay performed in an ionic liquid†
Sheila N.
Baker
a,
Eric B.
Brauns
b,
T. Mark
McCleskey
a,
Anthony K.
Burrell
a and
Gary A.
Baker‡
*a
aChemistry Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA
bChemistry Department, University of Idaho, Moscow, ID 38344, USA
First published on 6th June 2006
Abstract
We describe the first example of immunoanalysis performed within an ionic liquid with minimal deleterious effect; our results bode well for the development of second-generation biosensors, particularly in applications involving poorly water soluble analytes including pesticides, phospholipids, and illicit drugs.
The enzymatic reactions that support life often take place in an essentially non-aqueous environment such as a phospholipid bilayer or a crowded intracellular environment characterized by steric exclusion (i.e., “macromolecular crowding”) from inert solutes which may comprise as much as 50% of the total volume. Nonetheless, the unequivocal experimental demonstration that many (if not most) enzymes will in fact perform perfectly well in organic solvents with no macroscopic aqueous phase present was nothing short of revolutionary in the field of biocatalysis.1,2 Sometimes far less than a monolayer of water remains bound to the enzyme's surface under these conditions. The ability to conduct enzymatic reactions in non-aqueous or low water media was clearly a significant step forward for transformations involving substrates with poor water solubility. Other benefits include reductions in or elimination of expensive water removal processes, unwanted aqueous side reactions including hydrolysis, molecular memory impediments, and microbial contamination. Better activities, chemo-, regio-, or enantioselectivities, and operational stabilities are frequently reported as well. Similar advantages have been shown to extend to immunosensing within organic phases as illustrated in work by Russell et al.3
The choice of solvent with a reduced water activity may take several forms including neat organic solvents or mixtures, reverse micellar systems, supercritical fluids, fluorous phases, and ionic liquids. The latter have emerged as intriguing solvents for enzymology or biorecognition given their unique properties such as involatility, ionic conductivity, broad liquidus, and property designability. Early examples of the application of ionic liquids (ILs) toward biotransformation include a whole cell Rhodococcus R312 catalyzed two phase biotransformation of 1,3-dicyanobenzene to 3-cyanobenzene;4 lipase-catalyzed kinetic resolution of 1-phenylethanol;5 formation of Z-aspartame using thermolysin;6 lipase-catalyzed alcoholysis, ammoniolysis and perhydrolysis reactions;7 and the endoproteinase-catalyzed resolution of homophenylalanine ethyl ester.8 Some advantages of using ILs as solvents for enzymatic catalysis, beyond those cited above, are increased mean molecular weights of polymer products, enhanced solubility of polar substrates (e.g., carbohydrates),9 reduced emissions, ease of recycling, and enzyme thermostabilization,10 to name a few.
In addition, ILs have proven to be exceptional and versatile solvents in a host of analytical and chemosensing strategies.11 On the other hand, we are aware of no prior studies of their use as assay solvents or co-additives in any type of immunochemical application. We now report, for the first time, that the high-affinity hapten recognition function of an antibody is preserved both in free aqueous solution containing a large volume fraction of IL (up to 10–75 vol%) and for antibodies immobilized on solid supports and immersed in neat IL. Given the widespread interest in IL/water co-solvent mixtures as reaction media for biocatalysis, we focused our initial attention on the water-miscible prototypical IL 1-butyl-3-methylimidazolium tetrafluoroborate, [bmim][BF4]. In this paper, we report on the equilibrium binding affinity of intact polyclonal anti-BODIPY® FL antibodies from rabbit (immunoglobulin G (IgG) fraction; Invitrogen). This particular system was selected because BODIPY FL is excitable in the visible spectral region (“FL” denotes fluorescein-like emission), has a quantum yield close to unity, is very photostable, and can serve simultaneously as the hapten and the fluorescent reporting unit,12 the characteristics of which change substantially as BODIPY FL becomes specifically bound to anti-BODIPY FL.
Typical homogeneous fluorescence quenching immunoassay (FQIA) results for this system, obtained in phosphate buffer (PB; 100 mM, pH 8.0), are shown in Fig. 1a. In this case, anti-BODIPY/BODIPY association is accompanied by a red-shift from 510 to 519 nm and a significant degree of maximum quenching (Qm ≈ 87%) of the initial, unligated BODIPY fluorescence intensity; i.e., Qm = 1 − (Fmin/F0) where F0 and Fmin are the total integrated fluorescence intensities for identical analytical concentrations of free and antibody-bound BODIPY, respectively. Similar spectral behavior was exhibited during anti-BODIPY/BODIPY binding in the entire range of composition of [bmim][BF4]/PB mixtures preceding an upper turbidity limit near 80 vol% IL. For instance, 50 vol% [bmim][BF4] in PB showed a Qm value of ∼ 77% and a 5-nm red shift to 518 nm at binding saturation (Fig. S1, ESI†). In a control experiment, we confirmed that when challenged with the non-target fluorophores rhodamine 6G (R6G) and Texas Red (TR), anti-BODIPY produced no measurable quenching (Fig. S2). Taken together, our results demonstrate that soluble anti-BODIPY antibodies do indeed retain both their high binding affinity and recognition selectivity when dissolved in PB containing up to 75% [bmim][BF4]. Moreover, the similar polarity experienced by the antibody-bound BODIPY ligand suggests that the hydrogen bond interactions within the IgG combining site are not significantly perturbed by the presence of IL. This is further supported by Fig. S3 which shows that both the emission maximum and center-of-gravity (COG) for BODIPY in 50 vol% [bmim][BF4] approach, at binding saturation, their respective values in PB.
![Progression in the steady-state emission profile for BODIPY FL in PB resulting from 457-nm excitation as anti-BODIPY FL is added (a); raw data were corrected for blank signal and volume changes. FQIA binding isotherms for different vol%
[bmim][BF4] in PB (b). The solid curves are least squares fits to the data using eqn. (1) for A
= 66 nM.](/image/article/2006/CC/b606473f/b606473f-f1.gif) | ||
| Fig. 1 Progression in the steady-state emission profile for BODIPY FL in PB resulting from 457-nm excitation as anti-BODIPY FL is added (a); raw data were corrected for blank signal and volume changes. FQIA binding isotherms for different vol% [bmim][BF4] in PB (b). The solid curves are least squares fits to the data using eqn. (1) for A = 66 nM. | ||
Determination of the equilibrium binding affinity (Kf) for this system was permitted by following the net decrease in integrated fluorescence intensity (F0 − F) as a function of added anti-BODIPY at fixed BODIPY concentration. The resulting FQIA binding isotherms were then fitted using the following equation describing bimolecular association
 | (1) |
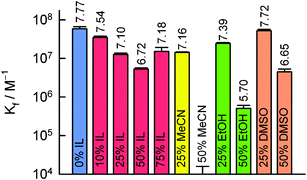 | ||
| Fig. 2 Recovered equilibrium binding constants (Kf) for anti-BODIPY/BODIPY complexation in various solvent systems. Standard deviations of triplicates in a single experiment are shown as error bars. The value for log Kf is placed above each bar. | ||
Encouraged as we were by this result, we were unfortunately unable to reliably estimate binding constants for PB systems containing much over 75% [bmim][BF4] due to the onset of opalescence and, ultimately, sedimentation of PB salts. In order to demonstrate antibody–hapten binding in 100% IL, we devised an experiment employing an array of anti-BODIPY “capture” antibodies immobilized onto a (3-aminopropyl)diethoxymethyl silane (APDEMS) self-assembled monolayer (SAM)§ pattern generated on glass. Because it offers easy access to micron resolution photo-patterning, we employed spatially-directed UV illumination/aminolysis13 to surface template a pre-existing APDEMS SAM,¶ as summarized schematically in Fig. 3. After coupling antibodies to the SAM pattern using reductive amination,|| the anti-BODIPY bioarrays were immersed in [bmim][X] (X = BF4−, Tf2N−, TfO−, or PF6−), each 100 nM in the BODIPY FL hapten, and incubated for 1.5 h at 37 °C followed by gentle rinsing with a laminar flow of pure IL to remove any loosely adsorbed tracer. For all four ILs investigated, the resulting fluorescence images showed high contrast and excellent uniformity (Fig. 3, inset). Importantly, no binding was observed when the IgG immunosurfaces were exposed to R6G or TR demonstrating that the arrays of immobilized antibodies maintained their biological integrity and high specificity in pure IL, suffering only minor activity losses (Kf > 106 M−1). In fact, anti-BODIPY IgG/BODIPY binding in neat [bmim][X] results in patterns that are equally uniform and stable as those measured in PB (Fig. S6), with a conservative estimate of the specific/nonspecific ratio of ∼ 50. Given that “free” fluorescent hapten is universally much more emissive than the IgG-bound form in FQIA, the fact that ILs give reduced non-specific binding/sorption (in unpatterned regions) is an important and completely unanticipated advantage of ILs as immuno-solvents.
![Schematic illustration of SAM photolithographic patterning via masked exposure to light from a mercury lamp (step 1) and subsequent antibody coupling to fabricate immunosurfaces (steps 2–4). Lower left: Representative epifluorescence microscopy image showing spatially selective BODIPY binding in neat [bmim][Tf2N]. Scale bar represents 25 µm. See ESI for full details.](/image/article/2006/CC/b606473f/b606473f-f3.gif) | ||
| Fig. 3 Schematic illustration of SAM photolithographic patterning via masked exposure to light from a mercury lamp (step 1) and subsequent antibody coupling to fabricate immunosurfaces (steps 2–4). Lower left: Representative epifluorescence microscopy image showing spatially selective BODIPY binding in neat [bmim][Tf2N]. Scale bar represents 25 µm. See ESI for full details. | ||
The underlying reasons for the slightly lowered affinity remain speculative but possible factors include reversible antibody conformational changes stemming from alterations in hydration level, Coulombic forces, or local dipolarity; or low-affinity binding of IL components to the antibody surface. Yet another option, given the inherent and distinctive segmental flexibility of the IgG molecule,12,14 is a matrix effect associated with dampened protein dynamics arising from solvent ordering and the relatively high viscosity of the IL and its mixtures with PB. The reduced mobility within [bmim][BF4]-rich aqueous mixtures is reflected in the precipitous drop in the fluorescence correlation spectroscopy (FCS)-determined diffusion coefficient (Dt) measured for BODIPY FL from 305 µm2 s−1 in PB to just 22 µm2 s−1 in 75 vol% [bmim][BF4]. For comparison, the mean Dt was only ca. 1.5 to 2.0 µm2 s−1 for similarly sized fluorescent probes in anhydrous [bmim][PF6].15 Corroborative evidence in support of this notion is further provided by the sharp recovery in Kf to the anticipated level as the [bmim][BF4] system is diluted with PB. It has been shown previously that antibody–hapten binding3 and enzyme specificity16 and reactivity17 in non-aqueous systems are related to the hydrophobicity of the organic solvent. It is then worth noting that the ET(30) value drops steadily from 62.8 kcal mol−1 for PB to 55.7 kcal mol−1 for 75% [bmim][BF4]. The observed behavior may be partially attributable to reduced hydrophilicity, however, we note that ET(30) = 52.4 and 45.4 kcal mol−1 for neat EtOH and MeCN/DMSO, respectively. In any case, it is important to realize that the measured binding affinities reported here are fully adequate for practical immunoassay.12,18,19
In conclusion, this report demonstrates that free antibody binding affinity can be almost completely retained for a significant volume fraction of [bmim][BF4] dissolved within PB as well as for supported antibodies subjected to pure IL as solvent. It also provides the sole example of bioreceptor action within an IL to date. These results hold compelling possibilities for advancing biosensors targeting a range of analyte species including traditionally difficult lipophilic targets of key importance in pharmacology, petrochemistry, food science, environmental monitoring, and homeland defense.19
G.A.B. acknowledges support via Frederick Reines and Eugene Wigner Fellowships. We thank Andrew Dattelbaum and Matt Ferris for kind technical assistance. This work is dedicated to Professor Raymond T. O'Donnell on the occasion of his retirement.
Notes and references
- Methods in Non-Aqueous Enzymology, ed. M. N. Gupta, Birkhäuser Verlag: Basel, Switzerland, 2000 Search PubMed.
- A. M. Klibanov, Chemtech, 1986, 16, 354 CAS; J. S. Dordick, Enzyme Microb. Technol., 1989, 11, 194 CrossRef CAS; M. N. Gupta, Eur. J. Biochem., 1992, 203, 25 CAS; L. Kvittingen, Tetrahedron, 1994, 50, 8253 CrossRef CAS.
- A. J. Russell, L. J. Trudel, P. L. Skipper, J. D. Groopman, S. R. Tannenbaum and A. M. Klibanov, Biochem. Biophys. Res. Commun., 1989, 158, 80 CAS.
- S. G. Cull, J. D. Holbrey, V. Vargas-Mora, K. R. Seddon and G. J. Lye, Biotechnol. Bioeng., 2000, 69, 227 CrossRef CAS.
- S. H. Schofer, N. Kaftzik, P. Wasserscheid and U. Kragl, Chem. Commun., 2001, 425 RSC.
- M. Erbeldinger, A. J. Messiano and A. J. Russell, Biotechnol. Prog., 2000, 16, 1129 CrossRef CAS.
- R. M. Lau, F. van Rantwijk, K. R. Seddon and R. A. Sheldon, Org. Lett., 2000, 2, 4189 CrossRef CAS.
- H. Zhao, R. G. Luo and S. V. Malhotra, Biotechnol. Prog., 2003, 19, 1016 CrossRef CAS.
- For an excellent overview see: U. Kragl, M. Eckstein and N. Kaftzik, in Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH: Weinheim, 2003; pp. 336–347 Search PubMed.
- S. N. Baker, T. M. McCleskey, S. Pandey and G. A. Baker, Chem. Commun., 2004, 940 RSC; K. Fujita, D. R. MacFarlane and M. Forsyth, Chem. Commun., 2005, 4804 RSC.
- G. A. Baker, S. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800 RSC; S. Pandey, Anal. Chim. Acta, 2006, 556, 38 CrossRef CAS.
- G. A. Baker, S. Pandey and F. V. Bright, Anal. Chem., 2000, 72, 5748 CrossRef; C. M. Cardona, J. Alvarez, A. E. Kaifer, T. D. McCarley, S. Pandey, G. A. Baker, N. J. Bonzagni and F. V. Bright, J. Am. Chem. Soc., 2000, 122, 6139 CrossRef CAS.
- A. M. Dattelbaum, M. L. Amweg, L. E. Ecke, C. K. Yee, A. P. Shreve and A. N. Parikh, Nano Lett., 2003, 3, 719 CrossRef; A. M. Dattelbaum, S. N. Baker and G. A. Baker, Chem. Commun., 2005, 939 RSC.
- R. Luedtke, C. S. Owen and F. Karush, Biochemistry, 1980, 19, 1182 CrossRef CAS; R. M. Watt, J. N. Herron and E. W. Voss, Jr., Mol. Immunol., 1980, 17, 1237 CrossRef CAS; M. A. Doody, G. A. Baker, S. Pandey and F. V. Bright, Chem. Mater., 2000, 12, 1142 CrossRef CAS.
- J. H. Werner, S. N. Baker and G. A. Baker, Analyst, 2003, 128, 786 RSC.
- T. Sakurai, A. L. Margolin, A. J. Russell and A. M. Klibanov, J. Am. Chem. Soc., 1988, 110, 7236 CrossRef CAS.
- J. D. Groopman, L. J. Trudel, P. R. Donahue, A. Marshak-Rothstein and G. N. Wogan, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 7728 CAS; A. Zaks and A. M. Klibanov, J. Biol. Chem., 1988, 263, 8017 CAS.
- The Immunoassay Handbook, ed. D. Wild, Elsevier Ltd: London, 3rd edn, 2005 Search PubMed.
- H. H. Weetall, J. Immunol. Methods, 1991, 136, 139 CrossRef CAS; S. Matsuura, Y. Hamano, H. Kita and Y. Takagaki, J. Biochem., 1993, 114, 273 CAS; L. Braco, Mikrochim. Acta, 1995, 120, 231 CAS; S. J. Setford, Trends Anal. Chem., 2000, 19, 330 CrossRef CAS; S. Dong and B. Wang, Electroanalysis, 2002, 14, 7 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: IL synthesis, detailed experimental procedures, and supporting Figs. S1–S6. See DOI: 10.1039/b606473f |
| ‡ Present address: Chemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA. E-mail: bakerga1@ornl.gov |
| § Monolayers formed by trialkoxysilanes are not strictly “self-assembled”, because the surface coupling is covalent (irreversible), but it is common to refer to them as SAMs as they share several of the traits of “true” self-assembled monolayers. |
| ¶ Wettability studies on the amino-terminated APDEMS SAMs showed advancing contact angles with water of 62 ± 2°. The hysteresis between advancing and receding contact angle was ca. 32°, which indicates that the monolayers are highly disordered. It is thus possible in future work to increase the stability of the monolayers and to optimize antibody orientation using protein A/G which both have a well known affinity for the Fc (fragment-crystallizable) tail region of IgG. |
| || Complete experimental protocols for SAM formation, photo-patterning, and bioconjugation are provided in the ESI. |
| This journal is © The Royal Society of Chemistry 2006 |
