Highlights
First published on 2nd December 2005
Abstract
Markus Hölscher reviews some of the recent literature in green chemistry
Sugar catalysts for the production of biodiesel
The transformation of diesel from vegetable oil suffers from the lack of an ecologically friendly catalyst. In its present form the process for the esterification of higher fatty acids uses H2SO4, which is both highly energy consumptive and chemically wasteful. Other materials such as Nafion or sulfonated carbonized naphthalene are either very expensive and have low activity or they are too soft and leach out during the liquid-phase reactions, leading also to a significant loss of activity. A collaboration of researchers from Tokyo, Yokohama and Ibaraki (Toda, Takagaki, Okamura, Kondo, Hayashi, Domen and Hara) recently investigated the possibility of carbonizing D-glucose at low temperatures, followed by sulfonation to generate an alternative catalyst for biodiesel production.1 The material obtained consists of sheets of amorphous carbon with hydroxyl and carboxyl and also SO3H groups in high density. As anticipated the solid is an appropriate catalyst for the esterification of the vegetable-oil constituents oleic acid and stearic acid, as was deduced from the high activity (more than half of the activity of liquid sulfuric acid) outperforming many other known solid catalysts for this reaction. Neither did the catalyst lose its activity nor was a leaching of SO3H detectable in repeated reactions at temperatures between 80 and 180 °C. Apart from the production of biodiesel the material might have potential for other acid catalyzed reactions that await a more sustainable process than the ones in use currently.Asymmetric transfer hydrogenation of imines with brønsted acids
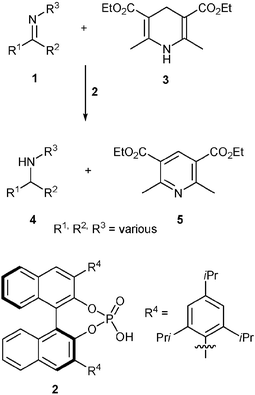
Knowledge about asymmetric transfer hydrogenations of ketimines is scarce, although the resulting products are chiral amines, which are industrially relevant building blocks. As the interest in organocatalytic reactions with chiral acids is growing, it was obvious to envision the use of chiral phosphoric acids in such reactions. List and coworkers from the Max-Planck-Institute of Coal Reserach, Mülheim, have very recently demonstrated the high potential of this approach.2A ketimine (1) is protonated by the acid catalyst (2), while the hydrogen centres are transferred from a Hantzsch ester (3) generating the desired chiral amine (4) and the corresponding pyridine (5). In a catalyst screening for evaluating the most promising catalyst structure it was found that modifications of 2 at the 3,3′-positions of the binaphthol core using the 2,4,6-iso-propylphenyl rest yielded a catalyst capable of generating high ee values. However, the conversion of a test substrate remained disappointingly low. Interestingly simple tuning of the reaction conditions improved the result significantly generating a catalyst system useful for the transfer hydrogenation of a large variety of ketimines with ee values up to 93% and yields of up to 98%.
Boehmite nanofibers as hosts for Rhodium nanoparticles—hydrogenation of arenes under mild conditions
Nanoparticles attract increasing attention for applications in catalysis. Mild hydrogenations of arenes are one of the fields in which these nanoparticles can be applied successfully. Though many different methods for the synthesis of nanoparticles have been developed, often these techniques suffer from difficult synthetic procedures, while generating nanoparticles of low stability and low activity. Park et al. from Pohang university, Korea, recently reported about the incorporation of Rhodium nanoparticles in a boehmite matrix, yielding stable, easy recyclable and highly active catalysts for the hydrogenation of various arenes.3 The simple synthesis consists of the mixing of RhCl3 hydrate with 2-butanol and Al(O-sec-Bu)3 with subsequent trapping of the material in boehmite by gelation with water. According to TEM images the boehmite nanofiber structure is maintained with Rh-nanoparticles residing inside the pores. The inner surface of the material amounts to ca. 600 m2 g−1 as calculated from BET adsorption measurements. The material was tested as a catalyst for the hydrogenation of a variety of arenes (benzene, anisole, toluene, phenol, ethyl benzoate, 1-phenylethanol, xylenes, cresols, naphthalene, to name a few) with yields of 100%, while operating either in solvents or solventless. Under a pressure of 1 atm of H2 in many cases the reactions did not require any heating and yielded the desired hydrogenated products in reaction times typically varying between 0.5 and 5 h.Homogeneous catalysis meets biocatalysis—Papain modified Rh-phosphites as hydrogenation catalysts
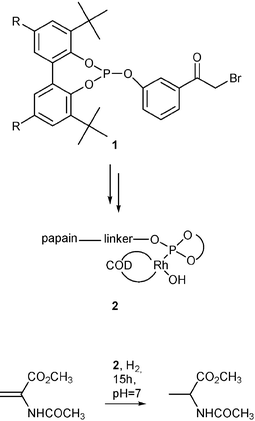
Asymmetric catalysis is considered as one of the key technologies for atom economic and highly selective transformations which by appropriate tuning (right choice of the environmentally benign solvents and reaction engineering) generally offers the possibility for the creation of sustainable chemical processes. However, due to practical considerations (e.g. time-to-market pressure) asymmetric catalysis has not evolved yet as the method of choice for industry. In an attempt to generate novel alternatives for the traditional homogeneous catalysts de Vries et al. have recently added an interesting example to the combined bio-metal-catalytic approach.4 They reasoned that a transition metal bound to an easily synthesizable, however achiral ligand, which in turn is anchored to readily available enzymes (which are chiral inherently) might yield an active and selective catalyst for asymmetric transformations.In a first set of experiments they have synthesized phosphite 1, which can be coupled to the enzyme papain, which has a highly nucleophilic SH-group usable for covalent binding of the phosphite. Additionally catalysts of this type have to be hydrolytically stable, a feature which is provided by the presence of bulky groups near the phosphorous centre. The resulting compound—a hybrid of a phosphorous ligand and an enzyme—is reacted with an appropriate Rhodium complex yielding the desired metallo-enzyme 2. As anticipated 2 is an active catalyst for the hydrogenation of 2-acetamidoacrylate yielding the corresponding alanine derivative with 100% conversion of the reactant and 100% product selectivity. However, no enantioselectivity was observed at all, which might be a result of the chiral environment of the enzyme being too far away from the catalytically active metal centre. Though disappointing at a first glance the approach is highly desirable due to the readily available starting materials.
Kenneth G. Hancock Memorial Student Award in Green Chemistry
Outstanding examples of studies or research by undergraduate and graduate students that address the scope and objectives of green chemistry can be honored by the Kenneth G. Hancock Memorial Student Award in Green Chemistry by the Green Chemistry Institute of the American Chemical Society. The prize is awarded annually and includes not only a one-time cash award of $1000 but also participation in the prestigious Presidential Green Chemistry Challenge Awards Ceremony in Washington, DC. The deadline for applications is February 1, 2006. Details can be found under http://chemistry.org/greenchemistryinstitute,, but naturally the prize focuses on alternative synthetic pathways for green chemistry, use of alternative reaction conditions and the design of alternative chemicals of less toxicity than of compounds currently in use.Green Chemistry Institute Sabbatical/Fellow Program
Sabbaticals or fellowships for professionals active in sustainable chemistry can be supported by the Green Chemistry Institute of the American Chemical Society. Applicants must provide a project proposal in an area relevant to green chemistry and support is granted for assistant, associate and full professors. Also junior and senior level professionals from industry and government can apply. The duration of the the fellowship is one academic year and the deadline for the 2006 applications is February 1. See http://chemistry.org/greenchemistryinstitute for more detailed information on the program.Green chemistry—it's in the books
Green chemistry is everywhere but no one writes about it?! Provocative but wrong. Having grown aware of the important consequences man-made chemical production brings to the planet, chemists from around the world have not only started making chemical processes sustainable many years ago (at least in some parts of the world), they have also considered the importance of bringing the ideas and fundamentals of sustainable chemical production into classrooms, management offices and—last not least—into the minds of the ones that take political decisions. Admittedly the process did not start from science alone, it needed the help of the growing public concern, but here it is: Knowledge about green chemical technology, renewable resources, waste management, sustainable consumption, supercritical fluids and future challenges (and many many more relevant topics) is brought together by the many books and articles that have been written by distinguished experts in the fields. The world-wide-web helps a lot here. Under www.chemsoc.org/pdf/gen/Books.pdf there is a list citing titles, authors, publishers and ISBN numbers of many interesting books about the field.Institute for Green Oxidation Chemistry
The Institute for Green Oxidation Chemistry is located at Carnegie Mellon University, Pittsburgh, USA. Three major areas were definded by the institute which need substantial modifications to improve on the development of sustainable technologies. Firstly, renewable energy technologies with a high emphasis on solar technologies should be at the core for the production of electricity in an industrial society. The institute aims to develop technologies for converting solar energy into chemical energy and then back to electrical energy. Secondly, chemical feedstocks should stem from renewable sources and thirdly, the institute carries out research to minimize the production of pollutants accompanying chemical processes. Learn more about the institute at http://www.chem.cmu.edu/groups/Collins/.References
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS.
- S. Hoffmann, A. M. Seayad and B. List, Angew. Chem., 2005, 17, 7590–7593 CrossRef.
- I. S. Park, M. S. Kwon, N. Kim, J. S. Lee, K. Y. Kang and J. Park, Chem. Commun., 2005, 5667–5669 RSC.
- L. Panella, J. Broos, J. Jin, M. W. Fraaije, D. B. Janssen, M. Jeronimus-Stratingh, B. L. Feringa, A. J. Minnaard and J. G. de Vries, Chem. Commun., 2005, 5656–5658 RSC.
| This journal is © The Royal Society of Chemistry 2006 |