The high-yield synthesis, spectroscopic and structural determination of three new uranium(IV) and thorium(IV)
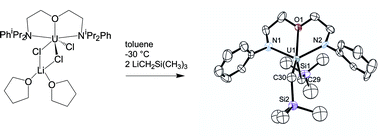
“ate” complexes supported by three different diamido ether ligands are reported. The reaction of Li2[2,6-iPr2PhN(CH2CH2)]2O (Li2[DIPPNCOCN]) with 1 equiv. of UCl4 in THF generates [DIPPNCOCN]UCl3Li(THF)2
(1), while reaction in toluene/ether gives salt-free [DIPPNCOCN]UCl2·½C7H8
(2), which was identified by paramagnetically shifted 1H NMR. Reaction of 0.5 equiv. of {[tBuNON]UCl2}2
([tBuNON]
=
[(CH3)3CN(Si(CH3)2)]2O2−) with 3.5 equiv. LiI in toluene and a minimal amount of THF results in [tBuNON]UI3Li(THF)2
(3) and is very similar in structure to 1. {[MesNON]ThCl3Li(THF)}2
(4), a dimeric complex with a Th2Li2Cl6 core, is prepared by reaction of Li2[2,4,6-Me3PhN(Si(CH3)2)]2O (Li2[MesNON]) with ThCl4 in THF. The analogous reaction in toluene did not yield the salt-free complex but rather a sterically crowded diligated compound, [MesNON]2Th (5), which was also structurally characterized. Complex 5 was prepared rationally by reacting 2 equiv. Li2[MesNON] with ThCl4 in toluene. The reaction of 1 and 3 with 2 equiv. of LiCH2Si(CH3)3 generates the stable, salt-free organoactinides [DIPPNCOCN]U(CH2Si(CH3)3)2
(6) and [tBuNON]U(CH2Si(CH3)3)2
(7). Complex 6 was structurally characterized. These reactions illustrate the viability of “ate” complexes as useful synthetic precursors.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...