Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells†
Raymond Wai-Yin
Sun
a,
Rong
Chen
a,
Nancy P.-Y.
Chung
b,
Chi-Ming
Ho
a,
Chen-Lung Steve
Lin
*b and
Chi-Ming
Che
*a
aDepartment of Chemistry and Open Laboratory of Chemical Biology of the Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, China. E-mail: cmche@hku.hk; Fax: (+852) 2857-1586
bDepartment of Surgery, The University of Hong Kong Medical Centre, Queen Mary Hospital, Pokfulam Road, Hong Kong, China
First published on 16th September 2005
Abstract
Silver nanoparticles fabricated in Hepes buffer exhibit potent cytoprotective and post-infected anti-HIV-1 activities toward Hut/CCR5 cells.
Metal nanoparticles have been attracting increasing attention due to their important applications in a number of subject areas such as catalysis and nanoscale electronics.1 Recently, much effort has been devoted to develop biomedical applications of metal nanoparticles. While significant advances in biological labeling have been made,2 few therapeutic applications of metal nanoparticles have been reported in the literature. A notable example is the anti-microbial properties of silver nanoparticles, which have been used for wound healing.3a To our knowledge, the anti-viral properties of metal nanoparticles remain an undeveloped area which is potentially of medicinal interest.3b
There are numerous methods for generating silver nanoparticles with different shapes and sizes.4 In this work, we found that silver nanoparticles could be readily fabricated using Hepes buffer. A typical reaction was by dissolving AgNO3 (2–5 mM) in Hepes buffer (0.1 M, pH 7.4) at room temperature for 120 h or under refluxing condition for 4 h. Formation of the nanoparticles was affected by buffer acidity, since no silver nanoparticle was formed when the pH was adjusted to <5. Fig. 1 shows transmission electron microscopy (TEM, A) and high-resolution transmission electron microscopy (HRTEM, B) images of the silver nanoparticles. These nanoparticles are uniformly distributed with particle size varied from 5 to 20 nm (with average diameter about 10 nm). A spot-profile energy-dispersive X-ray analysis (EDX) showed the presence of strong signals from the silver atoms together with Cu atom signal from the copper grid and weaker signals that are due to C atoms (Fig. S2, ESI†).
 | ||
| Fig. 1 The TEM (A) and the HRTEM (B) images of silver nanoparticles. | ||
The powder X-ray diffraction (XRD) pattern taken from a larger quantity of sample suggested that the silver nanoparticles generated in Hepes buffer existed in face-center-cubic phase. As depicted in Fig. S3 (ESI†), the presence of intense peaks corresponding to 2θ values of (111), (200), (220), and (311) in the powder X-ray diffraction pattern agrees with those values reported in literature (JCPDS No. 04-0783). The silver nanoparticles exhibit an absorption peak at 410 nm (Fig. S4, ESI†), which is close to the reported data.
Stability is an important issue of silver nanoparticles for therapeutic applications. Previous studies showed that silver nanoparticles were unstable in solution and would easily aggregate with average particle size >40 nm or at high concentration.5 To maintain adequate solution stability, the size of the silver nanoparticles prepared in this study was confined to 5 to 20 nm in diameter. These nanoparticles exhibited excellent stability at 50 µM in Hepes buffer; no significant spectral change was observed over 3 days at 37 °C based on UV-vis spectroscopy. However, at 1 mM level, silver precipitation with concomitant UV spectral changes was found after 3 days incubation at 37 °C. Similarly, silver nanoparticles prepared in citrate buffer by using traditional NaBH4 protocol also demonstrated good solution stability at 50 µM, but poor solution stability at 1 mM level.4c
Human serum albumin (HSA) is the most abundant plasma protein in circulatory system. Previous reports showed that this serum protein could be used to stabilize a variety of metal nanoparticles.6 In this study, HSA (2 mM, at physiologically relevant level) was employed to stabilize silver nanoparticles. Based on UV-vis spectroscopy, we found that in the presence of HSA, no significant spectral change of the silver nanoparticles with concentration up to 1 mM was observed at 37 °C for 7 days (Fig. S5, ESI†). Recently, Shen and co-workers had demonstrated hysteresis effect of the interaction between serum albumins and silver nanoparticles (60 nm).7 By means of UV-vis absorption titration, we also found that the silver nanoparticles would interact with HSA (Δλmax = +4 nm; hyperchromicity = 3%, Fig. S6, ESI†). TEM images showed that there was a change in size from 10 to ∼20 nm when the silver nanoparticles were treated with HSA for 3 days (Fig. S7, ESI†); nevertheless, no silver precipitation was observed.
The post-infected anti-viral [HIV-1(BaL)] activities of silver nanoparticles (10 nm, fabricated in Hepes buffer) toward Hut/CCR5 cells were evaluated herein. The viral contents were determined by measuring p24 antigen production in various cell cultures 3 days after infection (Fig. 2).8 As compared to the vehicle control, silver nanoparticles (0.5, 5, and 50 µM) prepared in Hepes showed dose-dependent anti-retrovirus activities and exhibited high potency at 50 µM (98%) in inhibiting HIV-1 replication. Similar inhibitory properties were found for the silver nanoparticles (10 nm) fabricated in citrate solution with NaBH4 as reducing agent. For comparison, the anti-HIV-1 activities of gold nanoparticles (10 nm, fabricated in Hepes buffer) were also examined. As shown in Fig. 2, the gold nanoparticles (10 nm) showed relatively low anti-HIV-1 activities (6–20%) as compared to that of silver nanoparticles.
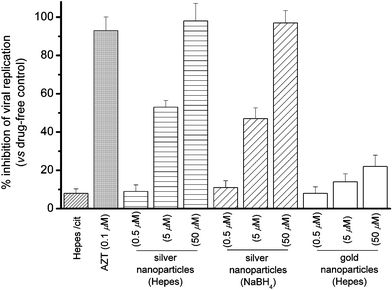 | ||
| Fig. 2 Percentage inhibition of HIV-1(BaL) replication in Hut/CCR5 cells (3 days) by silver and gold nanoparticles. | ||
Human T-cell is known to undergo apoptotic cell death after HIV infection,9 while successful anti-HIV agents/regimens would significantly drop the viral load and hence reduce apoptosis. In this study, we evaluated the cytoprotective activity of silver nanoparticles toward HIV-1 infected Hut/CCR5 cells by TUNEL (terminal uridyl-nucleotide end labeling) assays after the 3 day treatment. Plots of flow cytometric data (Fig. 3) showed that silver nanoparticles at 5 µM would reduce the percentage of apoptotic cells from 49 to 35%. There is a significantly drop (from 49% to 19%) in percentage at the effective anti-HIV-1 concentration (50 µM) as compared to that of the vehicle control. We reason that the silver nanoparticles inhibit viral replication in Hut/CCR5 cells and hence reduce HIV-associated apoptosis.
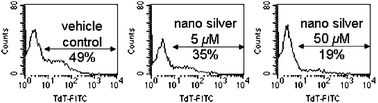 | ||
| Fig. 3 TUNEL assay showed that silver nanoparticles exhibit cytoprotective activities toward HIV-1 infected Hut/CCR5 cells. | ||
To confirm whether the silver nanoparticles were endowed with selective antiviral activities instead of killing the host Hut/CCR5 cells, the cytotoxicity was determined in parallel by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.10 Fig. S8 (ESI†) shows that these nanoparticles did not show acute cytotoxicity for the Hut/CCR5 cells, with >80% cell survival being registered at concentration up to 50 µM, which corresponds to the concentration required for effective anti-HIV-1 activity. These results highlight that the cytotoxicity of silver nanoparticles cannot account for the anti-viral properties. Furthermore, we also tested the acute cytotoxicity of the silver nanoparticles to human peripheral blood mononuclear cells (PBMC),11 the latter cells are the host for HIV-1 replication in vivo. Similar to the results that we tested on Hut/CCR5 cells, these nanoparticles showed low cytotoxicity to the normal PBMC (Fig. 4).
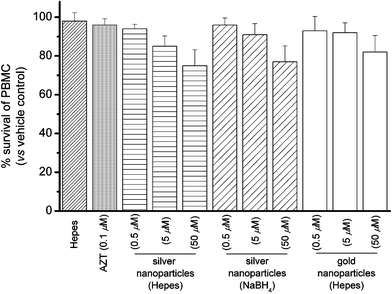 | ||
| Fig. 4 Percentage survival of peripheral blood mononuclear cells (PBMC) in the presence of silver and gold nanoparticles. | ||
To determine whether the HSA binding would alter the biological activities of the silver nanoparticles, the post-infected anti-HIV properties of the silver nanoparticles after incubation with HSA (HSA-nanoAg, in which [HSA] = 2 mM) were examined. A similar potency (i.e., 43 and 98% at 5 and 50 µM of silver nanoparticles, respectively) as compared to that of the silver nanoparticles alone was found. Indeed, HSA could be used as a carrier for a variety of substrates including amino acids, hormones, medicinal drugs, and even metal complexes and metal atoms from the site of adsorption to the site of action. As the HSA binding of the silver nanoparticles did not alter their post-infected anti-HIV-1 potency, this result highlights that HSA may also serve as a carrier for the silver nanoparticles to their biological targets (i.e., HIV infected cells).
HIV-1 reverse transcriptase (RT) is one of the major targets for anti-HIV drugs.12 In this study, the activities of silver nanoparticles (10 nm) toward HIV-1 RT inhibition were measured by using an ELISA method developed by Eberle and Seibl.13 Upon treatment of HIV-1 RT in lysis buffer (2 ng, 128.7 mL) with the silver nanoparticles (5 µM and 50 µM) at 37 °C, poor RT inhibition (∼8 and 17%, respectively) was observed after 30 min incubation as compared to RT inhibitor AZT-TP (i.e., 83% inhibition). Therefore, it appears that the silver nanoparticles inhibited HIV-1 replication via other yet-to-be-determined mechanism(s).
In summary, the cytoprotective and the post-infected anti-HIV activities of metal nanoparticles have been demonstrated. These nanoparticles were found to interact with human serum albumin, but meanwhile, their anti-viral properties were retained.
We acknowledge the financial support from the University of Hong Kong (University Development Fund) and the Areas of Excellence Scheme (AoE/P-10/01) administered by the University Grants Committee (HKSAR, China).
Notes and references
- (a) M. A. El-Sayed, Acc. Chem. Res., 2004, 37, 326 CrossRef CAS; (b) C.-M. Ho, W.-Y. Yu and C.-M. Che, Angew. Chem. Int. Ed., 2004, 43, 3303 CrossRef CAS; (c) H. Lang, R. A. May, B. L. Iversen and B. D. Chandler, J. Am. Chem. Soc., 2003, 125, 14832 CrossRef CAS; (d) L. N. Lewis, Chem. Rev., 1993, 93, 2693 CrossRef CAS.
- (a) S. R. Nicewarner-Pena, R. G. Freeman, B. D. Reiss, L. He, D. J. Pena, I. D. Walton, R. Cromer, C. D. Keating and M. J. Natan, Science, 2001, 294, 137 CrossRef CAS; (b) R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078 CrossRef CAS.
- (a) J. B. Wright, K. Lam, D. Hansen and R. E. Burrell, Am. J. Infect. Control, 1999, 27, 344 CAS; (b) J. L. Elechiguerra, J. L. Burt, J. R. Morones, A. Camacho-Bragado, X. Gao, H. H. Lara and M. J. Yacaman, J. Nanobiotechnol., 2005, 3, 6 Search PubMed.
- (a) P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940 CrossRef CAS; (b) Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS; (c) K. Esumi, A. Suzuki, A. Yamahira and K. Torigoe, Langmuir, 2000, 16, 2604 CrossRef CAS.
- F. Mafune, J.-Y. Kohno, Y. Takeda, T. Kondow and H. Sawabe, J. Phys. Chem. B, 2000, 104, 8333 CrossRef CAS.
- H. Xie, A. G. Tkachenko, W. R. Glomm, J. A. Ryan, M. K. Brennaman, J. M. Papanikolas, S. Franzen and D. L. Feldheim, Anal. Chem., 2003, 75, 5797 CrossRef CAS.
- X. Shen, Q. Yuan, H. Liang, H. Yan and X. He, Sci. China, 2003, 46, 387 Search PubMed.
- (a) A. Trkola, W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk and J. P. Moore, J. Virol., 1998, 72, 396 CAS; (b) S.-Y. Wong, R. W.-Y. Sun, N. P.-Y. Chung, C.-L. Lin and C.-M. Che, Chem. Commun., 2005, 3544 RSC.
- L. Meyaard, S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. M. Keet and F. Miedema, Science, 1992, 257, 217 CrossRef CAS.
- (a) T. Mosmann, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS; (b) C.-M. Che, R. W.-Y. Sun, W.-Y. Yu, C.-B. Ko, N. Zhu and H. Sun, Chem. Commun., 2003, 1718 RSC.
- R. W.-Y. Sun, W.-Y. Yu, H. Sun and C.-M. Che, ChemBioChem, 2004, 5, 1293 CrossRef CAS.
- H. Hitsuya, R. Yarchoan and S. Broder, Science, 1990, 249, 1533 CrossRef.
- J. Eberle and R. Seibl, J. Virol. Methods, 1992, 40, 347 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; TEM and HR-TEM images, spot-profile EDX analysis and XRD, and UV-vis spectrum of silver nanoparticles; UV-vis spectra of silver nanoparticles in Hepes buffer containing HSA; TEM image of silver nanoparticles after treatment with HSA; Percentage survival of Hut/CCR5 in the presence of silver and gold nanoparticles. See http://dx.doi.org/10.1039/b510984a/ |
| This journal is © The Royal Society of Chemistry 2005 |
