Photocatalytic overall water splitting under visible light by TaON and WO3 with an IO3−/I− shuttle redox mediator
Ryu
Abe
*a,
Tsuyoshi
Takata
b,
Hideki
Sugihara
a and
Kazunari
Domen
b
aNational Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, 305-8565, Japan. E-mail: r-abe@aist.go.jp; Fax: +81-29-861-4750; Tel: +81-29-861-9405
bFaculty of Engineering, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: domen@chemsys.t.u-tokyo.ac.jp; Fax: +81-3-5841-8838; Tel: +81-3-5841-1148
First published on 24th June 2005
Abstract
Photocatalytic water splitting into H2 and O2 under visible-light irradiation (λ > 420 nm) is demonstrated using the oxynitride Pt–TaON for H2 evolution and a Pt–WO3 catalyst for O2 evolution in an IO3−/I− shuttle redox-mediated system.
Photocatalytic water splitting into H2 and O2 by semiconducting catalysts has received much attention due to the potential of technology for the production of clean fuel H2 from water using solar energy. The development of a photocatalytic system that functions efficiently under visible light, representing almost half of the available solar spectrum, is therefore indispensable for the practical utilization of solar energy. The present authors have recently reported several stable non-oxide photocatalysts, (oxy)nitrides and oxysulfides, as potential candidates for visible light-induced water splitting.1–5 The valence bands of these materials are populated by N 2p and S 3p orbitals, respectively, mixed with O 2p, resulting in more negative valence band levels and smaller band gaps compared to oxide semiconductors. For example, oxynitride TaON with a band gap of 2.5 eV (absorption edge at 500 nm) has conduction and valence band edges of ca. −0.3 and +2.2 V vs. NHE (pH 0), respectively, sufficient for overall water splitting into H2 and O2.5 Our group has also recently developed a two-step water splitting system in which two different photocatalysts are combined using an IO3−/I− shuttle redox mediator.6–8 In this system, visible light can be utilized more efficiently than in conventional water splitting systems because the energy required to drive each photocatalytic system is reduced. However, the number of visible light-driven photocatalysts available for this system remains limited, and all of the existing materials are oxide semiconductors (e.g., WO3) or oxide-based semiconductors (e.g., metal cation-doped SrTiO3).7–9
The present paper reports the first application of an oxynitride photocatalyst to this two-step water splitting system with an IO3−/I− shuttle redox mediator. H2 evolution and IO3− production are shown to proceed over Pt–TaON in the presence of an I− electron donor under visible light, and stoichiometric water splitting into H2 and O2 under visible-light irradiation is demonstrated using a combination of Pt–TaON and Pt–WO3 photocatalysts.
TaON powder was prepared by heating Ta2O5 powder under an NH3 flow (20 ml min−1) at 1123 K for 15 h. WO3 powder (99.99%) was provided by Koujyundo Chemical, and other chemicals used in the experiments were purchased from commercial sources as guaranteed reagents and used without further purification. The TaON was loaded with Pt (0.3 wt%) by impregnation from aqueous {Pt(NH3)4}Cl2 solution followed by H2 reduction for 2 h at 573 K. In the case of WO3, Pt (0.5 wt%) was loaded by impregnation from aqueous H2PtCl6 solution followed by calcination in air for 1 h at 773 K. Photocatalytic reactions were carried out in a Pyrex reaction vessel connected to a closed-gas circulation system. The two photocatalytic powders (0.2 g of each) were suspended in distilled water under agitation using a magnetic stirrer, and the required amount of solute (e.g., NaI) was added to the suspension. The suspension was then thoroughly degassed. Argon gas (40 Torr) was introduced into the system, and the suspension was irradiated by a Xe lamp (300 W) fitted with a cut-off and water filters to eliminate light in the ultraviolet (UV) and infrared regions, respectively. The evolved gases were analyzed by on-line gas chromatography, and the abundance of I3− and IO3− anions produced by the reactions was determined by UV-visible absorption spectroscopy and ion chromatography, respectively.
Photocatalytic H2 evolution was found to occur over the Pt-loaded TaON catalyst in aqueous solution containing I− anions as an electron donor under visible light (λ > 420 nm), as shown in Fig. 1. The production of IO3− anions in the solution was also confirmed, while the accurate determination of the amount of IO3− was difficult because of the quite low concentration. Neither O2 nor N2 gases were evolved during this photoreaction. Therefore, the reactions taking place over the Pt–TaON photocatalyst under visible light are considered to be as follows:
| TaON + hν (λ > 420 nm) → e− + h+ | (1) |
| 2H+ + 2e− → H2 | (2) |
| I− + 6OH− + 6h+ → IO3− + 3H2O | (3) |
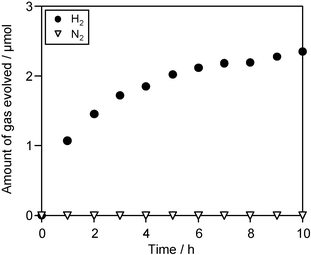 | ||
| Fig. 1 Time course of photocatalytic evolution of H2 using Pt (0.3 wt%)–TaON photocatalyst (0.2 g) suspended in a 5 mM NaI aqueous solution (pH 7 without adjustment) under visible light (λ > 420 nm). | ||
The results also indicate that the I− anions act as effective electron donors in the presence of the TaON photocatalyst. The rate of H2 evolution decreased gradually over time, and ceased when the concentration of IO3− anions produced in the solution reached a certain level. These results indicate that the backwards reaction described below, that is, the reduction of IO3− to I−, which is a thermodynamically favorable reaction, proceeds preferentially in place of the reduction of water when the concentration of IO3− reaches this threshold level.
| IO3− + 3H2O + 6e− → I− + 6OH− | (4) |
Consequently, the undesirable recycled reaction (IO3− ↔ I−, combination of eqns. (3) and (4)), which consumes electrons (e−) and holes (h+), eventually became dominant. A similar trend has been observed for H2 production over Pt–TiO2-anatase and Pt–SrTiO3 ∶ Cr/Ta (SrTiO3 codoped with Cr and Ta) photocatalysts in the presence of I− electron donors.6–8
Overall water splitting under visible light was attempted by combining the Pt–TaON catalyst with the Pt–WO3 photocatalyst for O2 evolution. As reported previously, the Pt–WO3 photocatalyst possesses a unique reactivity for the oxidation of water, allowing selective O2 evolution in the presence of IO3− electron acceptors even at low IO3− concentrations.7,8 As shown in Fig. 2, the combination of Pt (0.3 wt%)–TaON and Pt (0.5 wt%)–WO3 photocatalysts resulted in simultaneous H2 and O2 evolution (initial rates: H2, 24 µmol h−1; O2, 12 µmol h−1) from NaI aqueous solution (5 mM, pH 7 without adjustment) under visible light (λ > 420 nm). The quantum efficiency for overall water splitting was determined to be ca. 0.4% at 420 nm under the same conditions as for Fig. 2.10 The efficiency of the present system is comparable to other systems using the SrTiO3 based photocatalysts.7–9 No N2 evolution was detected during the photoreaction, and no gas evolution was observed in darkness. The reaction proceeded without notable deactivation even after 100 h, and the total amount of H2 gas evolved reached ca. 1.5 mmol, exceeding the stoichiometric amount of the photocatalysts (TaON, 0.95 mmol; WO3, 0.86 mmol) and I− (1.25 mmol) in the solution. No structural change of the photocatalysts could be detected by X-ray diffraction analysis after the photoreaction.
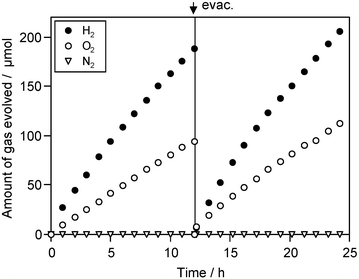 | ||
| Fig. 2 Time course of photocatalytic evolution of H2 and O2 using a mixture of Pt (0.3 wt%)–TaON (0.2 g) and Pt (0.5 wt%)–WO3 (0.2 g) suspended in a 5 mM NaI aqueous solution (pH 7 without adjustment) under visible light (λ > 420 nm). | ||
These results therefore demonstrate that overall water splitting proceeds by a two-step photo-excitation combined with a redox cycle between IO3− and I−, as shown in Fig. 3. The first step involves water reduction to H2 and I− oxidation to IO3− over Pt–TaON, and the second step involves IO3− reduction to I− and water oxidation to O2 over Pt–WO3. It should be noted that the rate of H2 evolution over the combined Pt–TaON/Pt–WO3 system was higher than over Pt–TaON alone (Fig. 1; ca. 1 µmol h−1). The prompt reduction of IO3− to I− over Pt–WO3 is considered to maintain a very low concentration of IO3− in the solution during the photoreaction, effectively suppressing the undesirable backwards reaction of IO3− reduction to I− over Pt–TaON, which cumulatively reduces the rate of H2 evolution when Pt–TaON is used alone.
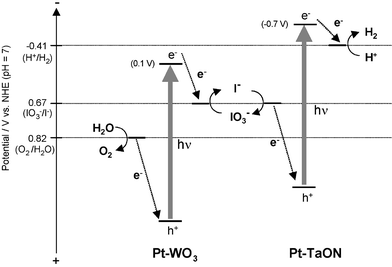 | ||
| Fig. 3 Speculated reaction mechanism for water splitting over Pt–TaON and Pt–WO3 with an IO3−/I− shuttle redox mediator. | ||
Although TaON exhibits high efficiency (QE = 34% at λ = 420–500 nm) for water oxidation to O2 in the presence of Ag+ as an electron acceptor,2 efficient O2 evolution over TaON in the presence of IO3− could not be achieved. This is considered to be attributable to the efficiency of I− as an electron donor over TaON, as indicated by the H2 evolution in the presence of I−. That is, photo-excited electrons over TaON reduce IO3− to I−, and the I− produced readily reacts with photo-generated holes over TaON, preventing O2 evolution.
In summary, the oxynitride TaON was applied for the first time to the two-step overall splitting of water through combination with WO3 using IO3−/I− as a shuttle redox mediator. The Pt-loaded TaON photocatalyst exhibited activity for water reduction to H2 and I− oxidation to IO3−, leading to stoichiometric overall water splitting under visible light irradiation by combination with Pt–WO3.
This work was supported by the Fund for Young Researchers from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and also was supported by the Solution-Oriented Research for Science and Technology (SORST) program of the Japan Science and Technology Corporation (JST).
Notes and references
- G. Hitoki, A. Ishikawa, T. Takata, J. N. Kondo, M. Hara and K. Domen, Chem. Lett., 2002, 736 CrossRef CAS.
- G. Hitoki, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Chem. Commun., 2002, 1968 RSC.
- A. Kasahara, K. Nukumizu, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Phys. Chem. B, 2003, 107, 791 CrossRef CAS.
- A. Ishikawa, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Am. Chem. Soc., 2002, 124, 13547 CrossRef CAS.
- W. J. Chun, A. Ishikawa, H. Fujisawa, T. Takata, J. N. Kondo, M. Hara, M. Kawai, Y. Matsumoto and K. Domen, J. Phys. Chem. B, 2003, 107, 1798 CrossRef CAS.
- R. Abe, K. Sayama, K. Domen and H. Arakawa, Chem. Phys. Lett., 2001, 344, 339 CrossRef CAS.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, Chem. Commun., 2001, 2416 RSC.
- R. Abe, K. Sayama and H. Sugihara, submitted.
- A. Kudo, H. Kato and I. Tsuji, Chem. Lett., 2004, 33, 1534 CrossRef CAS.
- Apparent quantum efficiencies (Φ) for overall water splitting were calculated by the equation Φ(%) = (4R/I) × 100, where R is the H2 evolution rate (molecules h−1) and I is the rate of incident photon absorption at 420 nm (photons h−1). I was determined using a bandpass filter (MZ0420, Asahi Spectra) and a thermopile power meter (S310, Scientech) to measure the number of photons reaching the solution. It should be noted that 4 photons are required for the production of one molecule of H2 in the case of two-step water splitting using a redox mediator, while 2 photons are required for the same process in conventional one-step water splitting.
| This journal is © The Royal Society of Chemistry 2005 |
