The synthesis, X-ray structure [monoclinic, P21/n, a
= 12.1690(1), b
= 7.8360(1), c
= 14.4250(1)
Å, β
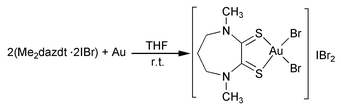
= 113.808(2)°], spectroscopic and electrochemical characterization of a new powerful reagent based on the iodine monobromide adduct of the N,N′-dimethylperhydrodiazepine-2,3-dithione (Me2dazdt) ligand, able to oxidize gold metal in a one-step reaction under mild conditions, is reported. The gold metal dissolution has been performed on gold powder, wires and Au/Ti thin layers. The oxidation product has been isolated and structurally characterised as [Au(Me2dazdt)Br2]IBr2 [monoclinic, C2/c, a
= 26.523(8), b
= 10.191(6), c
= 14.549(7)
Å, β
= 111.57(2)°]. The metal is essentially within a square planar geometry, Me2dazdt acts as an S,S chelating ligand and two bromide ligands complete the geometry around the metal. The IBr2− counteranion is essentially linear and shows I–Br bond lengths slightly asymmetric [Br(4)–I(1) 2.742(3), Br(3)–I(1) 2.682(2)
Å]. A comparison with the gold removal from Si/SiO2/Au/Ti thin layers of comparable thickness to that found in microelectronic devices, by using THF solutions of IBr and I2 adducts of the Me2dazdt donor, as well as the currently used I2/I− aqueous solutions, shows that these dihalogens-adducts produce a quantitative gold removal in shorter times and leaving the underlying layer perfectly clean, and are thus highly desirable as new etching agents in the gold-based technology of semiconductor devices.

 Please wait while we load your content...
Please wait while we load your content...