A new and a convenient route to enaminones and pyrazoles
Bogdan
Štefane
* and
Slovenko
Polanc
Faculty of Chemistry and Chemical Technology, University of Ljubljana, Aškerčeva 5, SI-1000 Ljubljana, Slovenia
First published on 7th January 2002
Abstract
A new method has been developed for the regioselective preparation of enaminones and pyrazoles from 1,3-diketonatoboron difluorides. The reactions proceed smoothly under mild reaction conditions, producing enaminones and pyrazoles in high yields.
Enaminones and related compounds, possessing the structural unit N–C
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C–Z (Z
C–Z (Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COR, CO2R, CN, etc.), are versatile synthetic intermediates in the construction of heterocycles.1 Pyrroles, oxazoles, pyridinones, quinolines, dibenzodiazepines, tetrahydrobenzoxazines, tetronic acids, and aza steroids have been prepared from enaminones.1,2 Enaminones are also valuable precursors for α-iodo enaminones,3 3-amino sugar derivatives,4 azo compounds,5β-aminoketones,6 as well as tetrahydropentalenes, tetrahydroindenes, and hexahydroazulenes.7 In addition, some of these compounds are pharmaceuticals possessing anticonvulsant8a–c
and analeptic8d activity, combined with low toxicity.
COR, CO2R, CN, etc.), are versatile synthetic intermediates in the construction of heterocycles.1 Pyrroles, oxazoles, pyridinones, quinolines, dibenzodiazepines, tetrahydrobenzoxazines, tetronic acids, and aza steroids have been prepared from enaminones.1,2 Enaminones are also valuable precursors for α-iodo enaminones,3 3-amino sugar derivatives,4 azo compounds,5β-aminoketones,6 as well as tetrahydropentalenes, tetrahydroindenes, and hexahydroazulenes.7 In addition, some of these compounds are pharmaceuticals possessing anticonvulsant8a–c
and analeptic8d activity, combined with low toxicity.
Enaminones are commonly prepared from amines and 1,3-diketones. The procedure leads to a single product if symmetrical 1,3-diketones9 or compounds containing two carbonyl groups of substantially different reactivity are employed,10 otherwise mixtures are obtained that are often difficult to separate. There is also a great variety of other methods for the preparation of unsymmetrical acyclic or cyclic enaminones.11 These methods involve several reaction steps, but they also suffer from the availability of the starting materials and, when 1,3-diketones and primary or secondary amines are used, they require prolonged heating at high temperatures and the azeotropic removal of water. A few years ago we have described a new synthetic route to pyrimidine N-oxides, starting from 1,3-diketones and carboxamide oximes.12 We found that reactions catalysed with BF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ·
·![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OEt2
gave pyrimidine N-oxides in low yields. Later on we discovered that low yields were due to the formation of 1,3-diketonatoboron difluorides. 1,3-Diketonatoboron difluorides, first described by Morgan and Tunstall,13 are known as valuable intermediates in organic synthesis.14 They can also be considered as protected 1,3-diketones.15
OEt2
gave pyrimidine N-oxides in low yields. Later on we discovered that low yields were due to the formation of 1,3-diketonatoboron difluorides. 1,3-Diketonatoboron difluorides, first described by Morgan and Tunstall,13 are known as valuable intermediates in organic synthesis.14 They can also be considered as protected 1,3-diketones.15
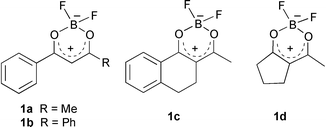
Herein we wish to report our preliminary results on the use of 1,3-diketonatoboron difluorides as starting materials for the regioselective synthesis of unsymmetrical enaminones and substituted pyrazoles. Initially, 1,3-diketonatoboron difluorides 1a–d were prepared in good yields by treatment of the corresponding 1,3-diketones with BF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ·
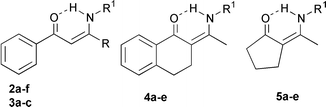
·![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OEt2 at room temperature (Table 1). Reactions of 1a–d with amino compounds afforded the products 2–5. The processes are highly regioselective and the products are formed in high yields.
OEt2 at room temperature (Table 1). Reactions of 1a–d with amino compounds afforded the products 2–5. The processes are highly regioselective and the products are formed in high yields.
The reactions of 2,2-difluoro-4-methylnaphtho[1,2-e]-1,3,2-dioxaborin and its [2,1-e] isomer with aniline have been reported.17 They give rise to the formation of the corresponding oxazaborines and not to the enaminones as described herein.
When we performed reactions with 1 or 2 equiv. of an amine, completion was not achieved within several hours. Under these conditions a small amount of the corresponding 1,3-diketones were detected in the reaction mixture. We found that at least 3 equiv. of the amine were required to avoid any side products. The above observation suggests that the coordination of boron in 1,3-diketonatoboron difluoride with an amine also plays a part in the successful termination of the reaction.
Benzoylacetonatoboron difluoride 1a was treated with ammonia, methylamine, isopropylamine, cysteamine, 3-picolylamine, and 3-amino-2,2-dimethylethanol to afford enaminones 2a–f (Table 2). It is worth mentioning that even cysteamine reacted with 1a to yield only the enaminone 2d. Comparable results were obtained employing difluorides 1b–c and the appropriate amines.
| Product | R | R1 | Reaction time/h | Yield (%)a | mp/°C |
|---|---|---|---|---|---|
a Isolated yields are given.
b Toluene.
c Lit.18a mp 144–145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
d Lit.18b mp 45–47 °C.
d Lit.18b mp 45–47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
e Lit.18c mp 56–58 °C.
e Lit.18c mp 56–58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
f Diethyl ether.
g Hexane–ethyl acetate.
h Hexane–diethyl ether.
i Diethyl ether. °C.
f Diethyl ether.
g Hexane–ethyl acetate.
h Hexane–diethyl ether.
i Diethyl ether.
|
|||||
| 2a | Me | H | 0.25 | 99 | 143–145bc |
| 2b | Me | Me | 0.25 | 99 | 68–69bd |
| 2c | Me | i-Pr | 0.5 | 98 | 57–58be |
| 2d | Me | CH2CH2SH | 0.5 | 98 | Oil |
| 2e | Me | 3-PyCH2 | 0.5 | 96 | Oil |
| 2f | Me | C(Me)2CH2OH | 0.5 | 79 | Oil |
| 3a | Ph | i-Pr | 0.5 | 91 | Oil18d |
| 3b | Ph | CH2CH2SH | 0.5 | 88 | Oil |
| 3c | Ph | 3-PyCH2 | 1 | 95 | 85–86f |
| 4a | — | H | 0.25 | 98 | 117–118g |
| 4b | — | Me | 0.25 | 99 | 53–54g |
| 4c | — | i-Pr | 1.5 | 99 | Oil |
| 4d | — | CH2CH2SH | 1.5 | 81 | Oil |
| 4e | — | 3-PyCH2 | 0.5 | 97 | 84–86h |
| 5a | — | H | 0.25 | 99 | 73–75i |
| 5b | — | Me | 0.25 | 99 | Oil |
| 5c | — | i-Pr | 0.25 | 95 | Oil |
| 5d | — | CH2CH2SH | 0.25 | 83 | 44–45i |
| 5e | — | 3-PyCH2 | 0.25 | 97 | Oil |
The problems associated with the use of low boiling amines have been overcome by the utilisation of a Lewis acid–amine complex.9 However, these reactions required prolonged heating in toluene and the removal of water. Furthermore, application of an excess of Lewis acid often resulted in the formation of a mixture of enaminones. In our procedure, ammonia or methylamine was passed over the stirred solution of 1,3-diketonatoboron difluoride for 5 min and the reaction was completed after an additional 15 min, giving only one regioisomer in excellent yield.
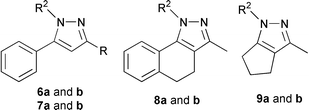
Our attention was then directed towards hydrazino compounds as nitrogen nucleophiles. Reaction of unsymmetrical 1,3-diketones with monosubstituted hydrazines can yield isomeric pyrazoles. It is known that benzoylacetone gives both 1,3-dimethyl-5-phenylpyrazole and 1,5-dimethyl-3-phenylpyrazole on reaction with methylhydrazine.19 There is also a report on pyrazole ring closure with methylhydrazine and phenylhydrazine, starting from benzoylacetone in heterogeneous media that resulted in a mixture of pyrazoles.20 When we reacted 1a with 3 equiv. of methylhydrazine we isolated only 1,3-dimethyl-5-phenylpyrazole (6a) in high yield. Similarly, phenylhydrazine furnished 1,5-diphenyl-3-methylpyrazole (6b). In addition, 1,3-diketonatoboron difluorides 1b–d reacted smoothly with hydrazines to offer a single regioisomer of 7–9Table 3.
| Product | R | R2 | Reaction time/h | Yield (%)a | mp/°C |
|---|---|---|---|---|---|
a Isolated yields are given.
b Lit.21a mp 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
c Hexane–diethyl ether.
d Lit.21c mp 59–60 °C.
c Hexane–diethyl ether.
d Lit.21c mp 59–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
e Ethanol.
f Lit.21d mp 138–139 °C.
e Ethanol.
f Lit.21d mp 138–139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
g Diethyl ether.
h Toluene. °C.
g Diethyl ether.
h Toluene.
|
|||||
| 6a | Me | Me | 0.5 | 95 | Oilb |
| 6b | Me | Ph | 2 | 83 | Oil21b |
| 7a | Ph | Me | 0.5 | 84 | 59–61cd |
| 7b | Ph | Ph | 2 | 89 | 138–140ef |
| 8a | — | Me | 0.5 | 88 | 165–167g |
| 8b | — | Ph | 0.5 | 91 | 87–89h |
| 9a | — | Me | 0.5 | 91 | 97–99h |
| 9b | — | Ph | 0.5 | 95 | 48–50c |
The structure elucidation of the enaminones and pyrazoles obtained was established by spectroscopic analyses, 1H, 13C NMR, HMQC, HMBC and NOESY spectra. The 1H-13C HMBC correlation and 2D NOE cross peaks clearly confirm the assigned regioisomers. The structure of compound 8b was also confirmed by an X-ray analysis (not reported).
In conclusion, we have described an efficient and regioselective approach to enaminones and pyrazoles. This methodology is of particular use, especially when one has to deal with a low boiling amine or an amine containing other nucleophilic functional groups (OH, SH). The reactions terminate under mild conditions in a short time with the formation of a single product that is easily isolable in high yields.
Experimental
General methods
Solvents and starting compounds were obtained from commercial sources (Fluka, Sigma and Aldrich). NMR spectra were recorded on a Bruker Avance 300 DPX spectrometer at 302 K. The HMBC spectra were obtained with 512 time increments and 32 scans per t1 increment. NOESY studies were acquired using the noesytp program on the DPX spectrometer. The mixing time was 100 ms and 32 accumulations were collected. Chemical shifts are reported relative to internal standard Me4Si. IR spectra were taken on BioRed FTS 3000MX instrument. Melting points were determined on a hot stage and are uncorrected. Microanalysis were performed on a Perkin-Elmer 2400 CHN Analyser. Mass spectra and high resolution mass measurements were acquired on a VG-Analytical Autospec EQ instrument.General procedure for the preparation of 1,3-diketonatoboron difluorides 1a–d
To a solution of the corresponding 1,3-diketone (1 equiv.) in toluene or dichloromethane (3 mL), boron trifluoride etherate (3 equiv.) was added at room temperature. The reaction mixture was allowed to stand at room temperature for the time noted in Table 1. Afterwards the reaction mixture was evaporated and the residue suspended in water (10 mL). Solid material was filtered off and recrystallised from toluene.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 Hz), 2.98 (t, 2H, J
7.4 Hz), 2.98 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 Hz), 7.28 (d, 1H, J
7.4 Hz), 7.28 (d, 1H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 Hz), 7.38 (dd, 1H, J1
7.5 Hz), 7.38 (dd, 1H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 Hz), 7.56 (ddd, 1H, J1
7.5 Hz), 7.56 (ddd, 1H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 Hz, J3
7.5 Hz, J3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 Hz), 8.11 (d, 1H, J
1.3 Hz), 8.11 (d, 1H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 Hz). 13C NMR (75 MHz, CDCl3): δ 21.6, 22.3, 27.5, 107.2,
127.6, 128.0, 128.3, 135.4, 143.5, 177.9, 189.59, 189.61. MS (EI, 70 eV, m/z
(%) 236 (M+, 100), 221 (91), 215 (55), 193 (65), 127 (58). HRMS (EI)
m/z calcd for C12H11BF2O2
7.5 Hz). 13C NMR (75 MHz, CDCl3): δ 21.6, 22.3, 27.5, 107.2,
127.6, 128.0, 128.3, 135.4, 143.5, 177.9, 189.59, 189.61. MS (EI, 70 eV, m/z
(%) 236 (M+, 100), 221 (91), 215 (55), 193 (65), 127 (58). HRMS (EI)
m/z calcd for C12H11BF2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 235.0857, found 235.0866. Anal. calcd. for C12H11BF2O2
: 235.0857, found 235.0866. Anal. calcd. for C12H11BF2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : C, 61.07; H, 4.70; found: C, 60.71; H, 4.50.
: C, 61.07; H, 4.70; found: C, 60.71; H, 4.50.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 2.27 (s, 3H), 2.67–2.78 (m, 4H).13C NMR (75 MHz, CDCl3): δ 20.1, 22.3, 25.2, 34.6, 113.0, 187.1, 199.3. MS (EI, 70 eV)
m/z
(%) 173 (M+, 23), 159 (100), 118 (42), 79 (35). HRMS (EI)
m/z calcd for C7H9BF2O2
7.8 Hz), 2.27 (s, 3H), 2.67–2.78 (m, 4H).13C NMR (75 MHz, CDCl3): δ 20.1, 22.3, 25.2, 34.6, 113.0, 187.1, 199.3. MS (EI, 70 eV)
m/z
(%) 173 (M+, 23), 159 (100), 118 (42), 79 (35). HRMS (EI)
m/z calcd for C7H9BF2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 173.0700, found 173.0707. Anal. calcd. for C7H9BF2O2
: 173.0700, found 173.0707. Anal. calcd. for C7H9BF2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : C, 48.33; H, 5.21; found: C, 48.08;
H, 5.26.
: C, 48.33; H, 5.21; found: C, 48.08;
H, 5.26.
General procedure for the synthesis of enaminones
To a stirred solution of a boron complex (1 equiv.) in acetonitrile (8 mL), amine (3 equiv.) was added at room temperature. In the case of ammonia or methylamine, the amine was passed over the stirred solution of a boron complex for 5 min at room temperature. The reaction mixture was stirred at room temperature for 0.25–1.5 h (see Table 2) and then evaporated to dryness. The residue was dissolved in dichloromethane (30 mL), washed with water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mL), dried over magnesium sulfate and evaporated to dryness. Products were purified in some cases by flash chromatography.
10 mL), dried over magnesium sulfate and evaporated to dryness. Products were purified in some cases by flash chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 Hz), 2.07 (s, 3H), 2.67–2.74 (m, 2H), 3.46–3.52 (m, 2H), 5.69 (s, 1H), 7.34–7.42 (m, 3H), 7.83–7.86 (m, 2H), 11.55 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 19.4, 25.0, 46.2, 92.6, 126.8, 128.1, 130.5, 140.1, 164.4, 187.9. MS (EI, 70 eV)
m/z
(%) 221 (M+, 42), 188 (69), 174 (76), 105 (100), 91 (77), 77 (67).
HRMS (EI)
m/z calcd for C12H15NOS: 221.0874, found 221.0882.
8.5 Hz), 2.07 (s, 3H), 2.67–2.74 (m, 2H), 3.46–3.52 (m, 2H), 5.69 (s, 1H), 7.34–7.42 (m, 3H), 7.83–7.86 (m, 2H), 11.55 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 19.4, 25.0, 46.2, 92.6, 126.8, 128.1, 130.5, 140.1, 164.4, 187.9. MS (EI, 70 eV)
m/z
(%) 221 (M+, 42), 188 (69), 174 (76), 105 (100), 91 (77), 77 (67).
HRMS (EI)
m/z calcd for C12H15NOS: 221.0874, found 221.0882.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4 Hz), 5.69 (s, 1H), 7.04–7.32 (m, 5H), 7.50–7.56 (m, 1H), 7.80–7.84 (m, 2H), 8.48 (d, 1H, J
6.4 Hz), 5.69 (s, 1H), 7.04–7.32 (m, 5H), 7.50–7.56 (m, 1H), 7.80–7.84 (m, 2H), 8.48 (d, 1H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4 Hz), 11.80 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 19.6, 48.6, 92.9, 121.1, 122.5, 127.0, 128.2, 130.6, 137.1, 140.3, 149.5, 157.2, 164.9, 188.1. MS (EI, 70 eV)
m/z
(%)
252 (M+, 37), 147 (100), 133 (72), 105 (52), 92 (50), 77 (55).
3.4 Hz), 11.80 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 19.6, 48.6, 92.9, 121.1, 122.5, 127.0, 128.2, 130.6, 137.1, 140.3, 149.5, 157.2, 164.9, 188.1. MS (EI, 70 eV)
m/z
(%)
252 (M+, 37), 147 (100), 133 (72), 105 (52), 92 (50), 77 (55).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 233.1416, found 233.1424.
: 233.1416, found 233.1424.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 Hz), 2.59–2.67 (m, 2H), 3.37–3.44 (m, 2H), 5.80 (s, 1H), 7.38–7.46 (m, 8H), 7.88–7.91 (m, 2H), 11.45 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 25.6, 47.7, 94.2, 127.2, 127.8, 128.3, 128.7, 129.7, 130.9, 135.5, 140.2, 166.6, 188.9. MS (EI, 70 eV)
m/z
(%) 283 (M+, 27), 250 (100), 236 (82), 105 (36). HRMS (EI)
m/z calcd for C17H17NOS:
283.1031, found 283.1041.
8.5 Hz), 2.59–2.67 (m, 2H), 3.37–3.44 (m, 2H), 5.80 (s, 1H), 7.38–7.46 (m, 8H), 7.88–7.91 (m, 2H), 11.45 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 25.6, 47.7, 94.2, 127.2, 127.8, 128.3, 128.7, 129.7, 130.9, 135.5, 140.2, 166.6, 188.9. MS (EI, 70 eV)
m/z
(%) 283 (M+, 27), 250 (100), 236 (82), 105 (36). HRMS (EI)
m/z calcd for C17H17NOS:
283.1031, found 283.1041.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.6 Hz), 5.87 (s, 1H), 7.18–7.22 (m, 1H), 7.33–7.53 (m, 8H), 7.53–7.57 (m, 1H), 7.87–7.90 (m, 2H), 8.36 (d, 1H, J
6.6 Hz), 5.87 (s, 1H), 7.18–7.22 (m, 1H), 7.33–7.53 (m, 8H), 7.53–7.57 (m, 1H), 7.87–7.90 (m, 2H), 8.36 (d, 1H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 Hz), 8.46–8.49 (m, 1H), 11.68 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 45.9, 94.5, 123.6, 127.1, 127.7, 128.3, 128.7, 129.8, 131.0, 134.1, 134.7, 135.1,
139.9, 148.6, 148.8, 166.6, 189.0. MS (EI, 70 eV)
m/z
(%) 314 (M+, 39), 223 (95), 149 (75), 105 (100), 77 (97). HRMS (EI)
m/z: calcd for C21H18N2O: 314.1419, found 314.1426.
1.8 Hz), 8.46–8.49 (m, 1H), 11.68 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 45.9, 94.5, 123.6, 127.1, 127.7, 128.3, 128.7, 129.8, 131.0, 134.1, 134.7, 135.1,
139.9, 148.6, 148.8, 166.6, 189.0. MS (EI, 70 eV)
m/z
(%) 314 (M+, 39), 223 (95), 149 (75), 105 (100), 77 (97). HRMS (EI)
m/z: calcd for C21H18N2O: 314.1419, found 314.1426.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 2.84 (t, 2H, J
7.2 Hz), 2.84 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 5.10 (br s, 1H), 7.15–7.37 (m, 3H), 7.99–8.02 (m, 1H), 10.88 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 21.6, 25.0, 29.6, 101.3, 126.9 (2C), 127.6, 131.3, 136.2, 142.0, 160.7, 186.1. MS (EI, 70 eV)
m/z
(%) 187 (M+, 100), 172 (14), 97 (13), 69 (15). HRMS (EI)
m/z
calcd for C12H13NO: 187.0997, found 187.1005.
7.2 Hz), 5.10 (br s, 1H), 7.15–7.37 (m, 3H), 7.99–8.02 (m, 1H), 10.88 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 21.6, 25.0, 29.6, 101.3, 126.9 (2C), 127.6, 131.3, 136.2, 142.0, 160.7, 186.1. MS (EI, 70 eV)
m/z
(%) 187 (M+, 100), 172 (14), 97 (13), 69 (15). HRMS (EI)
m/z
calcd for C12H13NO: 187.0997, found 187.1005.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4 Hz), 2.80 (t, 2H, J
6.4 Hz), 2.80 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4 Hz), 2.98 (d, 3H, J
6.4 Hz), 2.98 (d, 3H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1 Hz), 7.12–7.15 (m, 1H), 7.25–7.32 (m, 2H), 7.97–8.01 (m, 1H), 12.52 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.63, 25.0, 29.6, 30.2, 100.5, 126.3, 126.7, 127.2, 130.5, 136.5, 141.1, 164.7, 183.3. MS (EI, 70 eV)
m/z
(%) 201 (M+,
100), 186 (52), 56 (97). HRMS (EI)
m/z calcd for C13H15NO: 201.1154, found 201.1160.
5.1 Hz), 7.12–7.15 (m, 1H), 7.25–7.32 (m, 2H), 7.97–8.01 (m, 1H), 12.52 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.63, 25.0, 29.6, 30.2, 100.5, 126.3, 126.7, 127.2, 130.5, 136.5, 141.1, 164.7, 183.3. MS (EI, 70 eV)
m/z
(%) 201 (M+,
100), 186 (52), 56 (97). HRMS (EI)
m/z calcd for C13H15NO: 201.1154, found 201.1160.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1 Hz), 2.06 (s, 3H), 2.56 (t, 2H, J
6.1 Hz), 2.06 (s, 3H), 2.56 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6 Hz), 2.80 (t, 2H, J
7.6 Hz), 2.80 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6 Hz), 3.75–3.86 (m, 1H), 7.12–7.32 (m, 3H), 8.00–8.10 (m, 1H), 12.74 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.8, 24.1, 24.8, 29.5, 45.2, 100.1, 126.2, 126.6, 127.1, 130.4, 136.5, 140.9, 162.6, 183.0. MS (EI, 70 eV)
m/z
(%) 229 (M+,
100), 212 (70), 186 (86), 145 (40), 69 (85). HRMS (EI)
m/z calcd for C15H19NO: 229.1467, found 229.1475.
7.6 Hz), 3.75–3.86 (m, 1H), 7.12–7.32 (m, 3H), 8.00–8.10 (m, 1H), 12.74 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.8, 24.1, 24.8, 29.5, 45.2, 100.1, 126.2, 126.6, 127.1, 130.4, 136.5, 140.9, 162.6, 183.0. MS (EI, 70 eV)
m/z
(%) 229 (M+,
100), 212 (70), 186 (86), 145 (40), 69 (85). HRMS (EI)
m/z calcd for C15H19NO: 229.1467, found 229.1475.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 Hz), 2.02 (s, 3H), 2.56 (t, 2H, J
6.5 Hz), 2.02 (s, 3H), 2.56 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 2.67–2.81 (m, 4H), 3.49 (t, 2H, J
7.8 Hz), 2.67–2.81 (m, 4H), 3.49 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 Hz), 7.11–7.14 (m, 1H), 7.26–7.30 (m, 2H), 7.97–8.00 (m, 1H), 12.70 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.4, 24.4, 24.7, 28.9, 46.3, 100.5, 125.9, 126.1, 126.7, 130.2, 135.7, 140.6, 162.4, 183.5. MS (EI, 70 eV)
m/z
(%)
247 (M+, 46), 214 (58), 200 (100), 102 (49). HRMS (EI)
m/z calcd for C14H17NOS: 247.1031, found 247.1037.
6.5 Hz), 7.11–7.14 (m, 1H), 7.26–7.30 (m, 2H), 7.97–8.00 (m, 1H), 12.70 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.4, 24.4, 24.7, 28.9, 46.3, 100.5, 125.9, 126.1, 126.7, 130.2, 135.7, 140.6, 162.4, 183.5. MS (EI, 70 eV)
m/z
(%)
247 (M+, 46), 214 (58), 200 (100), 102 (49). HRMS (EI)
m/z calcd for C14H17NOS: 247.1031, found 247.1037.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 Hz), 2.83 (t, 2H, J
6.3 Hz), 2.83 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 Hz), 4.56 (d, 2H, J
6.3 Hz), 4.56 (d, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 Hz), 7.14–7.36 (m, 4H), 7.65–7.68 (m, 1H), 7.97–8.00 (m, 1H), 8.52–8.57 (m, 2H), 12.88 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.9, 24.8, 29.3, 44.9, 101.5, 123.8, 126.3, 126.6, 127.1, 130.8, 133.9, 134.7, 135.9, 144.1, 148.7, 149.0, 162.6,
184.5. MS (EI, 70 eV)
m/z
(%) 278 (M+, 87), 261 (70), 186 (77), 92 (100), 65 (54). HRMS (EI)
m/z calcd for C18H18N2O: 278.1419, found 278.1420.
6.0 Hz), 7.14–7.36 (m, 4H), 7.65–7.68 (m, 1H), 7.97–8.00 (m, 1H), 8.52–8.57 (m, 2H), 12.88 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 14.9, 24.8, 29.3, 44.9, 101.5, 123.8, 126.3, 126.6, 127.1, 130.8, 133.9, 134.7, 135.9, 144.1, 148.7, 149.0, 162.6,
184.5. MS (EI, 70 eV)
m/z
(%) 278 (M+, 87), 261 (70), 186 (77), 92 (100), 65 (54). HRMS (EI)
m/z calcd for C18H18N2O: 278.1419, found 278.1420.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 1.91 (s, 3H), 2.30 (d, 2H, J
7.2 Hz), 1.91 (s, 3H), 2.30 (d, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 2.51 (d, 2H, J
7.2 Hz), 2.51 (d, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 5.13 (br s, 1H), 9.23 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 20.4, 20.6, 27.2, 39.0, 103.4, 155.8, 203.9. MS (EI, 70 eV)
m/z
(%) 125 (M+, 92), 97 (33), 82 (40), 69 (100). HRMS (EI)
m/z calcd for C7H11NO: 125.0841,
found 125.0846.
7.2 Hz), 5.13 (br s, 1H), 9.23 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 20.4, 20.6, 27.2, 39.0, 103.4, 155.8, 203.9. MS (EI, 70 eV)
m/z
(%) 125 (M+, 92), 97 (33), 82 (40), 69 (100). HRMS (EI)
m/z calcd for C7H11NO: 125.0841,
found 125.0846.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 2.53 (t, 2H, J
7.2 Hz), 2.53 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 Hz), 2.93 (d, 3H, J
7.2 Hz), 2.93 (d, 3H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 Hz), 10.26 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 15.6, 20.4, 27.9, 29.5, 39.0, 102.3, 159.5, 201.4. MS (EI, 70 eV)
m/z
(%) 139 (M+, 98), 124 (38), 82 (66), 61 (100). HRMS (EI)
m/z calcd for C8H13NO: 139.0997, found 139.0997.
5.0 Hz), 10.26 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 15.6, 20.4, 27.9, 29.5, 39.0, 102.3, 159.5, 201.4. MS (EI, 70 eV)
m/z
(%) 139 (M+, 98), 124 (38), 82 (66), 61 (100). HRMS (EI)
m/z calcd for C8H13NO: 139.0997, found 139.0997.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 Hz), 1.82 (tt, 2H, J1
6.3 Hz), 1.82 (tt, 2H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 1.96 (s, 3H), 2.31 (t, 2H, J
7.8 Hz), 1.96 (s, 3H), 2.31 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 2.51 (t, 2H, J
7.8 Hz), 2.51 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 3.73 (sep, 1H, J
7.8 Hz), 3.73 (sep, 1H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 Hz), 10.36 (br s, 1H).13C NMR (75 MHz, CDCl3): δ 15.7, 20.3, 23.9, 28.0, 39.1, 44.5, 101.9, 157.6, 201.1. MS (EI, 70 eV)
m/z
(%) 167 (M+,
24), 105 (37), 69 (100). HRMS (EI)
m/z calcd for C10H17NO: 167.1310, found 167.1316.
6.3 Hz), 10.36 (br s, 1H).13C NMR (75 MHz, CDCl3): δ 15.7, 20.3, 23.9, 28.0, 39.1, 44.5, 101.9, 157.6, 201.1. MS (EI, 70 eV)
m/z
(%) 167 (M+,
24), 105 (37), 69 (100). HRMS (EI)
m/z calcd for C10H17NO: 167.1310, found 167.1316.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.7 Hz), 1.79–1.89 (m, 2H), 1.96 (s, 3H), 2.33 (t, 2H, J
8.7 Hz), 1.79–1.89 (m, 2H), 1.96 (s, 3H), 2.33 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 2.53 (t, 2H, J
7.8 Hz), 2.53 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz), 2.64–2.71 (m, 2H), 3.44 (dt, 2H, J1
7.8 Hz), 2.64–2.71 (m, 2H), 3.44 (dt, 2H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.6 Hz), 10.47 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 15.9, 20.4, 25.3, 27.9, 39.1, 46.0, 103.2, 157.7, 202.4. MS (EI, 70 eV)
m/z
(%) 185 (M+,
25), 152 (45), 138 (100), 61 (32). HRMS (EI)
m/z calcd for C9H15NOS: 185.0874, found 185.0875.
6.6 Hz), 10.47 (br s, 1H). 13C NMR (75 MHz, CDCl3): δ 15.9, 20.4, 25.3, 27.9, 39.1, 46.0, 103.2, 157.7, 202.4. MS (EI, 70 eV)
m/z
(%) 185 (M+,
25), 152 (45), 138 (100), 61 (32). HRMS (EI)
m/z calcd for C9H15NOS: 185.0874, found 185.0875.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.3 Hz), 1.92 (s, 3H), 2.35 (t, 2H, J
7.3 Hz), 1.92 (s, 3H), 2.35 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.3 Hz), 2.55 (t, 2H, J
7.3 Hz), 2.55 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.3 Hz), 4.47 (d, 2H, J
7.3 Hz), 4.47 (d, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.6 Hz), 7.27–7.31 (m, 1H), 7.61–7.64 (m, 1H), 8.52–8.55 (m, 2H), 10.67 (br s, 1H).13C NMR (75 MHz, CDCl3): δ 16.0, 20.4, 27.9, 39.2, 44.3, 103.9, 123.8, 134.2, 134.6, 148.5, 148.9, 157.6, 203.0.
MS (EI, 70 eV)
m/z
(%) 216 (M+, 94), 159 (57), 124 (65), 107 (43), 92 (100). HRMS (EI)
m/z calcd for C13H16N2O: 216.1263, found 216.1271.
6.6 Hz), 7.27–7.31 (m, 1H), 7.61–7.64 (m, 1H), 8.52–8.55 (m, 2H), 10.67 (br s, 1H).13C NMR (75 MHz, CDCl3): δ 16.0, 20.4, 27.9, 39.2, 44.3, 103.9, 123.8, 134.2, 134.6, 148.5, 148.9, 157.6, 203.0.
MS (EI, 70 eV)
m/z
(%) 216 (M+, 94), 159 (57), 124 (65), 107 (43), 92 (100). HRMS (EI)
m/z calcd for C13H16N2O: 216.1263, found 216.1271.
General procedure for the preparation of pyrazoles
To a stirred solution of a boron complex (1 mmol) in acetonitrile (5 mL), methylhydrazine or phenylhydrazine (3 mmol) was added at room temperature. The reaction mixture was stirred at room temperature for the time mentioned in Table 3 and then evaporated to dryness. The residue was treated with ice-cold water (15 mL), then the solid material was filtered off and rinsed with water, yielding pure pyrazoles.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.9 Hz), 2.98 (t, 2H, J
6.9 Hz), 2.98 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.9 Hz), 4.40 (s, 3H), 7.28–7.40 (m, 2H), 7.60–7.63 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 10.2, 18.4, 30.2, 38.4, 117.6, 124.0, 124.2, 127.9, 130.0, 130.9, 139.3, 141.0, 141.7. MS (EI, 70 eV)
m/z
(%) 198 (M+, 100), 183 (45), 115 (22). HRMS (EI)
m/z calcd for C13H14N2O: 198.1157, found 198.1162.
6.9 Hz), 4.40 (s, 3H), 7.28–7.40 (m, 2H), 7.60–7.63 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 10.2, 18.4, 30.2, 38.4, 117.6, 124.0, 124.2, 127.9, 130.0, 130.9, 139.3, 141.0, 141.7. MS (EI, 70 eV)
m/z
(%) 198 (M+, 100), 183 (45), 115 (22). HRMS (EI)
m/z calcd for C13H14N2O: 198.1157, found 198.1162.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.9 Hz), 2.97 (t, 2H, J
6.9 Hz), 2.97 (t, 2H, J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.9 Hz), 6.83 (dd, 1H, J1
6.9 Hz), 6.83 (dd, 1H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz, J2
7.8 Hz, J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 Hz), 6.96 (ddd, 1H, J1
1.2 Hz), 6.96 (ddd, 1H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz, J3
7.8 Hz, J3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 Hz), 7.08 (ddd, 1H, J1
1.2 Hz), 7.08 (ddd, 1H, J1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J2
J2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 Hz, J3
7.8 Hz, J3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 Hz), 7.11–7.14 (m,
1H), 7.23–7.50 (m, 5H). 13C NMR (75 MHz, CDCl3): δ 11.7, 19.3, 30.7, 118.6, 123.1, 125.6, 126.3, 127.1, 127.4, 127.9, 128.6, 129.3, 137.2, 138.2, 140.9, 146.0. MS (EI, 70 eV)
m/z
(%) 260 (M+, 100), 245 (32), 218 (35), 115 (10). HRMS (EI)
m/z calcd for C18H16N2
1.2 Hz), 7.11–7.14 (m,
1H), 7.23–7.50 (m, 5H). 13C NMR (75 MHz, CDCl3): δ 11.7, 19.3, 30.7, 118.6, 123.1, 125.6, 126.3, 127.1, 127.4, 127.9, 128.6, 129.3, 137.2, 138.2, 140.9, 146.0. MS (EI, 70 eV)
m/z
(%) 260 (M+, 100), 245 (32), 218 (35), 115 (10). HRMS (EI)
m/z calcd for C18H16N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 260.1314, found 260.1308.
: 260.1314, found 260.1308.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 136.1001, found 136.1007.
: 136.1001, found 136.1007.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 Hz), 7.15–7.20 (m, 1H), 7.35–7.41 (m, 2H), 7.57–7.60 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 12.7, 22.4, 26.9, 31.0, 118.9, 125.3, 128.4, 129.3, 140.3, 143.9, 149.2. MS (EI, 70 eV)
m/z
(%) 198 (M+, 100), 156 (30), 77 (53). HRMS (EI)
m/z calcd for C13H14N2
1.8 Hz), 7.15–7.20 (m, 1H), 7.35–7.41 (m, 2H), 7.57–7.60 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 12.7, 22.4, 26.9, 31.0, 118.9, 125.3, 128.4, 129.3, 140.3, 143.9, 149.2. MS (EI, 70 eV)
m/z
(%) 198 (M+, 100), 156 (30), 77 (53). HRMS (EI)
m/z calcd for C13H14N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 198.1157, found 198.1162.
: 198.1157, found 198.1162.
Acknowledgements
The authors thank Dr Bogdan Kralj and Dr Dušan Žigon (Mass Spectrometry Centre, Jožef Stefan Institute, Ljubljana, Slovenia) for mass measurements. The Ministry of Education, Science and Sport of Slovenia (PO-0503-0103) is gratefully acknowledged for financial support.References
- (a) J. V. Greenhill, Chem. Soc., Rev., 1977, 6, 277 RSC; (b) P. Lue and J. V. Greenhill, Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Academic Press, New York, 1997, vol. 67, pp. 207–343. Search PubMed.
- (a) A. Alberola, L. A. Calvo, A. G. Ortega, M. C. S. Ruiz and P. Yustos, J. Org. Chem., 1999, 64, 9493 CrossRef CAS; (b) I. Chaaban, J. V. Greenhill and P. Akhtar, J. Chem. Soc., Perkin Trans. 1, 1979, 1593 RSC; (c) R. Augusti and C. Kascheres, J. Org. Chem., 1993, 58, 7079 CrossRef CAS; (d) A. Müller, A. Maier, R. Neumann and G. Maas, Eur. J. Org. Chem., 1998, 1177 CrossRef; (e) E. Bejan, H. Aït-Haddou, J. C. Daran and G. G. A. Balavoine, Eur. J. Org. Chem., 1998, 2907 CrossRef CAS; (f) M. N. Eberlin and C. Kascheres, J. Org. Chem., 1988, 53, 2084 CrossRef CAS; (g) A. W. Trautwein and G. Jung, Tetrahedron Lett., 1998, 39, 8263 CrossRef CAS.
- P. J. Campos, J. Arranz and M. A. Rodríguez, Tetrahedron Lett., 1997, 38, 8397 CrossRef CAS.
- (a) L. F. Tietze and E. Voß, Tetrahedron Lett., 1986, 27, 6181 CrossRef CAS; (b) L. F. Tietze, U. Hartfiel, T. Hübsch, E. Voß and J. Wichmann, Chem. Ber., 1991, 124, 881 CrossRef CAS.
- L. J. O. Figueiredo and C. Kascheres, J. Org. Chem., 1997, 62, 1164 CrossRef CAS.
- (a) P. F. Schuda, C. B. Ebner and T. M. Morgan, Tetrahedron Lett., 1986, 27, 2567 CrossRef CAS; (b) R. SanMartín, E. M. Marigorta and E. Domínguez, Tetrahedron, 1994, 50, 2255 CrossRef CAS.
- R. Aummann, I. Göttker-Schnetmann, R. Fröhlich, P. Saarenketo and C. Holst, Chem. Eur. J., 2001, 7, 711 CrossRef.
- (a) I. O. Edafiogho, C. N. Hinko, H. Chang, J. A. Moore, D. Mulzac, J. M. Nicholson and K. Scott, J. Med. Chem., 1992, 35, 2798 CrossRef CAS; (b) K. R. Scott, I. O. Edafiogho, E. L. Richardson, V. A. Farrar, J. A. Moor, E. I. Tietz, C. N. Hinko, H. Chang, A. El-Assadi and J. M. Nicholson, J. Med. Chem., 1993, 36, 1947 CrossRef CAS; (c) K. R. Scott, G. O. Rankin, J. P. Stables, M. S. Alexander, I. O. Edafiogho, V. A. Farrar, K. R. Kolen, J. A. Moor, L. D. Sims and A. D. Tonnu, J. Med. Chem., 1995, 38, 4033 CrossRef CAS; (d) Hoffmann-La Roche, Ger. Pat. (DRP), 1935, 614, 195; Chem. Abstr., 1935, 29, 59953.
- M. Azzaro, S. Geribaldi and B. Videau, Synthesis, 1981, 880 CrossRef CAS.
-
(a) N. M. D. Brown and D. C. Nonhebel, Tetrahedron, 1968, 24, 5655 CrossRef CAS;
(b) R. V. Singh and J. P. Tandon, J. Prakt. Chem., 1979, 151; R. V. Singh and J. P. Tandon, Chem. Abstr., 1979, 90,
214
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461h..
461h.. - (a) G. Sauvé and V. S. Rao, in Comprehensive Organic Functional Group Transformation, ed. A. R. Katritzky, O. Meth-Cohn and C. W. Rees, Pergamon, New York, 1995, vol. 2, pp. 744–747; Search PubMed; (b) W. J. Ebenezer and P. Wight, in Comprehensive Organic Functional Group Transformations, eds. A. R. Katritzky, O. Meth-Cohn and C. W. Rees, Pergamon, New York, 1995, vol. 3, pp. 261–269; Search PubMed.
- (a) B. Mlakar, B. Štefane, M. Kočevar and S. Polanc, Tetrahedron, 1998, 54, 4387 CrossRef CAS; (b) B. Mlakar, B. Štefane, M. Kočevar and S. Polanc, Heterocycles, 1998, 48, 961 Search PubMed.
- G. T. Morgan and R. B. Tunstall, J. Chem. Soc., 1924, 1963 Search PubMed.
-
(a) Y. L. Chow and X. Cheng, J. Chem. Soc., Chem. Commun., 1990, 1043 RSC;
(b) Y. L. Chow, S. P. Wu and X. Ouyang, J. Org. Chem., 1994, 59, 421 CrossRef CAS;
(c) Y. L. Chow, S.-S. Wang, C. I. Johansson and Z.-L. Liu, J. Am. Chem. Soc., 1996, 118, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725 CrossRef CAS.
725 CrossRef CAS. - (a) B. Staskun, J. Org. Chem., 1979, 44, 875 CrossRef CAS; (b) S. C. Mackay, P. N. Preston, S. G. Will and J. O. Morley, J. Chem. Soc., Chem. Commun., 1982, 395 RSC.
- (a) J. A. Durden and D. G. Crosby, J. Org. Chem., 1965, 30, 1684 CAS; (b) C. R. Hauser, F. C. Frostick and E. H. Man, J. Am. Chem. Soc., 1952, 74, 3231 CrossRef CAS; (c) P. Czerney, C. Igney, G. Haucke and H. Hartmann, Z. Chem., 1988, 28, 23 Search PubMed.
- J. A. VanAllan and G. A. Reynolds, J. Heterocycl. Chem., 1969, 6, 29 Search PubMed.
- (a) P. G. Baraldi, D. Simoni and S. Manfredini, Synthesis, 1983, 902 CrossRef CAS; (b) W. Walter and T. Fleck, Liebigs Ann. Chem., 1976, 670 Search PubMed; (c) G. Bartoli, C. Cimarelli, G. Palmieri, M. Bosco and R. Dalpazzo, Synthesis, 1990, 895 CrossRef CAS; (d) Y. P. Singh, P. Rupani, A. Singh, A. K. Rai, R. C. Mehrotra, R. D. Rogers and J. L. Atwood, Inorg. Chem., 1986, 25, 3076 CrossRef CAS.
- T. L. Jacobs, in Heterocyclic Compound, ed. R. C. Elderfield, John Wiley & Sons, New York, 1957, vol. 5, p. 45 Search PubMed.
- F. Texier-Boullet, B. Klein and J. Hamelin, Synthesis, 1986, 409 CrossRef CAS.
- (a) A. Alberola, L. A. Banuelos, P. Cuadrado, A. Gonzalez and F. J. Pulido, Org. Prep. Proced. Int., 1989, 21, 237 Search PubMed; (b) A. C. Kovelesky and H. J. Shine, J. Org. Chem., 1988, 53, 1973 CrossRef CAS; (c) R. Huisgen, H. Gotthardt and R. Crashey, Chem. Ber., 1968, 101, 536 Search PubMed; (d) R. S. Foote, C. F. Beam and C. R. Houser, J. Heterocycl. Chem., 1970, 7, 589 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2002 |