Personal exposures and microenvironmental concentrations of particles and bioaerosols
Mika
Toivola
*a,
Sari
Alm
a,
Tiina
Reponen
b,
Sirpa
Kolari
c and
Aino
Nevalainen
ad
aNational Public Health Institute, Division of Environmental Health, P.O. Box 95, FIN-70701 Kuopio, Finland. E-mail: Mika.Toivola@ktl.fi; Fax: +358 17 201155
bDepartment of Environmental Health, University of Cincinnati, P.O. Box 670056, Cincinnati, OH 45267-0056, USA
cVTT Building and Transport, P.O. Box 1804, FIN-02044 VTT, Finland
dDepartment of Health Evaluation Sciences, Penn State University College of Medicine, 600 Centerview Drive, Hershey, PA 17033, USA
First published on 9th January 2002
Abstract
The aim of this study was to compare the personal exposure to particles and bioaerosols with that measured by stationary samplers in the main microenvironments, i.e., the home and the workplace. A random sample of 81 elementary school teachers was selected from the 823 teachers working for two councils in eastern Finland for the winter time measurement period. Bioaerosol and other particles were collected on filters by button samplers using personal sampling and microenvironmental measurements in homes and workplaces. The 24-hour sampling period was repeated twice for each teacher. Particle mass, absorption coefficient of the filter and the concentration of viable and total microorganisms were analyzed from each filter. In this paper, the study design, quality assurance principles and results of particle and bioaerosol exposure are described. The results show that particle mass concentrations, absorption coefficient and fungi were higher in personal exposure samples than in home and workplace samples. Furthermore, these concentrations were usually lower in the home than in the workplace. Bacterial concentrations were highest in heavily populated workplaces, while the viable fungi concentrations were lowest in workplaces. The fungi and bacteria results showed high variation, which emphasises the importance of quality assurance (duplicates and field blanks) in the microbial field measurements. Our results indicate that personal exposure measurements of bioaerosols in indoor environments are feasible and supplement the information obtained by stationary samplers.
Introduction
The association between dampness, moisture and mould problems in buildings and adverse health effects has been well documented,1–5 but little is known about the personal exposure that may cause these health effects. Microbial contamination of buildings may produce high concentrations of fungi and bacteria in indoor air,6–8 especially in the size range 1.1–3.3 µm,9,10 but the airborne concentrations vary in time and space even within the same room.11–13 In addition, high levels of total volatile organic compounds (TVOC) and characteristic microbial metabolites among the volatile organic compounds have been detected in moisture damaged buildings.14,15 Preliminary evidence on the importance of microbial toxins as exposure agents has been found, although individual toxins have not been detected directly in indoor air.16,17 People living or working in mouldy buildings are not only exposed to biological aerosols, but also to other particles originating from indoor sources as well as from outdoor air. Many studies have shown that particles of ambient air are associated with many health effects, such as increased respiratory symptoms, decreased lung function, increased hospital emergency admissions and respiratory and cardiovascular mortality.18–20 In particular, traffic related fine particles have been reported to be harmful.21 However, 37% (in number terms) of all outdoor air particles larger than 0.2 µm appear to be of biological origin.22 The health importance of biological particles in relation to other particle materials is not known, although similar inflammatory responses in vitro are induced by both diesel particles and indoor bioaerosols.23,24Many studies have used ambient particle concentrations [total suspended particulates (TSP), particles smaller than 10 µm (PM10), particles smaller than 2.5 µm (PM2.5)] to assess exposure to particles. An increasing number of studies using personal monitors have shown that personal exposure is usually higher than the indoor or outdoor concentrations of particles measured with stationary samplers. The correlation between ambient concentration and personal exposure to particles depends on several factors, including the particle size. An increase in particle size has been shown to decrease this correlation.25 These studies, however, have not assessed the proportion of particles consisting of biological aerosols. The exposure to bioaerosols usually has been assessed by many indirect short time indoor stationary measurement methods, such as 10 min air sampling for viable microbes in the room.26 The results of stationary samples with a short sampling time correlate poorly with health effects and are probably only surrogate measures of real exposure.27,28 There is a clear need for more accurate methods of exposure assessment, especially personal exposure to bioaerosols.
Although various bioaerosols, such as fungi, bacteria and pollen, are present everywhere and are part of our normal environment, indoor bioaerosols have been a focus of interest because of their possible role in the development of respiratory health effects. Indoor exposures to bioaerosols are important from a health perspective because individuals spend most of their time indoors. The most important factors affecting the concentrations of fungi and bacteria indoors are moisture and dampness in buildings. In Finland, 50% of private houses have been assessed to have some signs of moisture that need attention or repair.29 Moisture problems are also prevalent in schools.30–32 However, bioaerosol concentrations measured as short time samples in schools are not especially high, even when moisture and mould problems are visually evident and symptoms are experienced by both school students and teachers.32–34 Therefore, the question of personal exposures remains the most critical missing link in understanding the potential connections between bioaerosol exposure and human health.
The aim of this study was to compare the personal exposure to particles and bioaerosols with the exposure measured by stationary samplers in the main microenvironments, i.e. the home and the workplace. The study was carried out among elementary school teachers between 1998 and 1999. Personal and microenvironmental measurements of particle, fungi and bacteria concentrations and light absorption coefficients of filters, a marker for elemental carbon, were conducted at home and in the workplace of teachers. In this paper, the study design, quality assurance principles and preliminary results of particle and bioaerosol exposure are described.
Materials and methods
Elementary school teachers (n = 823) from two cities in eastern Finland were asked to fill in a short screening questionnaire. It included questions about the home and work environments and health status of the individuals, including their willingness to participate in the study. The response rate of the screening questionnaire was 67% (n = 562). A randomly selected subsample of 81 teachers was asked to participate in the study, which consisted of two 24 h monitoring periods of personal exposure and microenvironmental concentrations of bioaerosols and other particles. The teachers were also asked to respond to more detailed questionnaires and to agree to undergo technical investigations in their home and workplace environments. The measurements were conducted in the winter (November 1998 to March 1999, November to December 1999) when snow cover minimised the concentrations of outdoor airborne microbes. The measurement periods were repeated on the same week day, at 2 week intervals, except in four cases when the second measurement period was 5 weeks later. The purpose of the short 2 week period between measurements was to minimise possible changes in the environment of the participants.Each personal exposure sample was collected during a 24 h sampling period by a personal exposure monitor (PEM) carried by the participant. The monitor was an aluminium briefcase including a modified pump (AFC 400S, BGI Inc., Waltham, MA, USA), a button sampler (SKC Inc., Eighty Four, PA, USA) and noise absorption material (total weight, 3.5 kg). The pump was light and capable of running for a 24 h sampling period with four D-size alkaline batteries. Microenvironmental samples were collected by a microenvironmental monitor (MEM). The monitor consisted of a pump (PQ 100, BGI Inc., Waltham, MA, USA) and a button sampler. The pump was provided with an autotimer and mass flow adjustment system and operated with household electricity or internal battery. Before each sampling period, the flow rate was preset to 4 L min−1 with a gas flow calibrator (Buck M-30, A.P. Buck Inc., Orlando, FL 32809, USA) in the laboratory with the filter to be used. After each sampling period, the flow rate was verified. The sampling period was 24 h in homes and 8 h during a working day in workplaces.
Bioaerosol and particle samples of personal exposure and microenvironmental measurements were collected with the button inhalable aerosol sampler (SKC Inc.) developed at the University of Cincinnati, Aerosol Research and Exposure Assessment Laboratory.35 The button sampler utilises a porous curved surface as the sampling inlet. The sampler can be used as both a personal and a stationary sampler. It has been designed to follow the ACGIH/CEN/ISO inhalable convention curve. Laboratory and field tests have shown that the button sampler has lower wind sensitivity, lower intersample variation of the measured concentration levels and higher uniformity of the particle deposits on the filters than samples obtained with the standard 37 mm cassette.35,36 In this study, the particle samples were collected on a 25 mm PVC filter (0.8 µm pore size; Millipore, Bedford, MA, USA).
The mass concentration of collected particles was measured by weighing the filters with a microbalance (MT-5, Mettler-Toledo AG, Greifensee, Switzerland) of 1 µg sensitivity before and after sampling, according to the procedure used in the Air Pollution Exposure Distributions of Adult Urban Populations in Europe (EXPOLIS) project.37,38 Before weighing, each filter was stabilised in a weighing room for at least 2 h.39 The weighing conditions in the room were observed by recording the temperature, relative humidity and air pressure during each weighing session. Each filter was deionised on both sides with a Po-210 deioniser (Staticmaster 1269 by Cahn Inc., USA) before weighing. The buoyance corrected mass concentration was calculated using 1.004 kg m−3 as the density of the filter.38
The light absorption coefficients of the PVC filters were measured with the Black Smoke Method, according to the ISO protocol,40 by a smokestain reflectometer (M43D, Diffusion Systems Limited, London, UK). Before each measurement session, the reflectometer was calibrated with a standard tile supplied by the manufacturer, adjusted with a clean filter to show unit 100. The absorption coefficient was measured on five points of each filter. To eliminate the effect of exterior light, the filters were measured in a dark chamber. The absorption coefficient, a (m−1), for the filter was calculated according to eqn. (1):
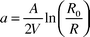 | (1) |
On the same day, after the weighing and reflectance measurements, the particles were extracted from the filters. The filters were instilled in 5 mL dilution buffer (distilled water with 42.5 mg L−1 KH2PO4, 250 mg L−1 MgSO4, 8 mg L−1 NaOH and 0.02% Tween 80) and the particles were removed from the filter by an ultrasonic bath (15 min, FinnSonic m03/m, Finland) and a shaker (15 min, IKA KS125 B, Germany). After extraction, the suspension was divided into two parts: the first part was used for microbiological analyses (2.1 mL) and the second part for toxicological analyses (2.9 mL, reported separately).
The total concentration of the collected microbes on the filters was determined by a direct counting method.41 The suspension (1 mL) was stained with acridine orange (Fluka Chemical, Switzerland) and filtered through a black filter (polycarbonate 0.2 µm, Whatman Nucleopore, USA). The filter was mounted on a microscope slide with non-fluorescence immersion oil and the microbes were counted under an epifluorescence microscope (Olympus BH-2, Olympus Optical Co., Tokyo, Japan). Forty randomly chosen fields were counted using 1000× magnification. Fungal spores and bacteria were distinguished from each other by their size.
The concentrations and genera of the viable microbes were determined by the cultivation method. The suspension was serially diluted (1∶10 and 1∶100) and cultured in duplicate on two fungal media [2% malt-extract agar (MEA, Biokar, France) and dichloran glycerol 18 agar (DG18, Oxoid, UK)] and on a bacterial medium [tryptone-yeast-glucose agar (TGY, Difco, USA)]. The fungal culture plates were incubated for 7 days at 25 °C. After incubation, the number of colonies was counted and the fungal genera were identified by morphology (will be reported separately). Bacterial plates were first incubated at 20 °C for 5 days, and the colonies were counted to obtain the concentration of total viable bacteria. The number of actinomycete colonies was counted after an additional 9 days of incubation.
To obtain comparable results, measurements with a sampling time of less than 75% of the demanded sampling time (24 or 8 h) or a sampling time of 24 h instead of 8 h were rejected. There were eight (5%) home samples, three (2%) personal measurement samples and nine (6%) workplace samples with an incorrect or too short measurement time. The numbers of rejected samples of total bacteria were 16 (10%) for home samples and 18 (11%) for personal and workplace samples. The reasons for rejection included drying of the sample or missing samples. In addition, one home sample for total fungi and one home sample for total and viable fungi and bacteria were accidentally destroyed during laboratory processing.
Standard operation procedures (SOP) were written for field procedures and laboratory analyses. Irregularities or deviations in the procedures were recorded. Duplicates and field blanks were collected to calculate detection limits, repeatability and possible sample contamination of the methods. Seven per cent of the actual samples were duplicates (36/486 measurements) and 6% were field blanks (30/486 measurements). The duplicates and field blanks were obtained and analysed side by side with the actual samples. The MEM duplicates and blanks were obtained from homes or workplaces of the participants, but the PEM duplicates and blanks were carried by the researchers. Detection limits (DL) for mass concentrations were calculated according to eqn. (2):
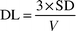 | (2) |
To evaluate the comparability of personal and microenvironmental monitors for the measurement of the mass concentration and filter absorption coefficient, these two monitor types were tested in parallel. All 10 PEMs and 11 MEMs used in this study were placed side by side in outdoor and indoor environments for 24 h.
The counting of total microbes was repeated twice with 35 samples to define the repeatability of counting. Sixteen samples were excluded because of a zero result. The difference in counting of 19 samples was not statistically significant (for fungi, P = 0.13; for bacteria, P = 0.83; non-parametric Wilcoxon signed rank test).
As part of the total design of the study, a nasal lavage fluid sample was taken from a subsample of 50 individuals after each sampling period. Immediately after both measurement periods, all of the participants were asked to fill in a 24 h time activity and exposure questionnaire to screen the events of the previous 24 h which could possibly have affected the exposure. The participants also completed an extensive background questionnaire covering details of health symptoms as well as home and workplace characterisation. After the measurement periods in both the home and workplace of each participant, a technical investigation was made by a civil engineer for signs of moisture or mould damage according to a checklist developed in previous studies by this research group.29 The results of the nasal lavage samples, symptom questionnaires and technical investigations will be reported separately.
To evaluate how well the subsample of 81 teachers participating in the study represents the population of teachers, the results of the short screening questionnaire were compared between the two populations using Pearson's chi-squared test. Differences between the personal, home and workplace concentrations of mass, fungi and bacteria were tested by non-parametric Kruskal–Wallis test and further analysed by Dunn's test.42 The differences between the absorption coefficients were tested by analysis of variance (ANOVA) and Dunnett's test. The Wilcoxon signed rank test was utilised to test the difference between field duplicates. Statistical analyses were performed with the program SPSS for Windows, version 9.0.
Results
There were no statistically significant differences in the characteristics between the exposure study subsample (n = 81) and all the respondents who filled in the short screening questionnaire (n = 562) (Table 1). A higher proportion of the exposure study subsample lived in suburban areas and single family or row houses compared with all the respondents. The exposure study subsample also reported more moisture damage and odour of mould at home and work related health problems than all the respondents. With respect to the respondents, 88% wanted to participate in the study, 2% no longer lived in the area when the measurements were made and 10% refused to participate in the study.| Respondents (n = 562) | Exposure (n = 81) | |
|---|---|---|
| a Missing information, 3. b Missing information, 1. c Missing information, 2. d During previous year. e Ever. f Multiple choices allowed. | ||
| Average age/yearsa | 43.2 | 43.7 |
| Women (%)b | 68 | 67 |
| Married (%)c | 81 | 83 |
| Self smoking (%)c | 8 | 9 |
| Some other smoking (%)c | 9 | 10 |
| Atopy (self or relatives) (%)c | 58 | 61 |
| Home characteristics (%) | ||
| Location | ||
| City centre | 23 | 19 |
| Suburban | 64 | 68 |
| Countryside | 13 | 14 |
| Single family or row house | 68 | 74 |
| Indoor air in the home | ||
| No harm of indoor air problems | 86 | 86 |
| Discomfort | 11 | 10 |
| Health problems | 4 | 6 |
| No moisture damage, mould growth or smell of mouldd | 81 | 75 |
| Moisture damaged | 14 | 17 |
| Mould growthd | 3 | 1 |
| Smell of mouldd | 5 | 9 |
| No repair of moisture damagee | 33 | 41 |
| Repair of moisture damagee | 21 | 20 |
| No moisture damagee | 46 | 39 |
| Indoor air of workplace (%) | ||
| No harm of indoor air problems | 39 | 35 |
| Discomfort | 42 | 44 |
| Health problems | 29 | 35 |
| Commuting to work in winter (%) f | ||
| Walk or cycle | 37 | 38 |
| Car | 65 | 67 |
| Bus | 13 | 9 |
The average age of the exposure subsample was 43.7 years and most of the participants were married women (Table 1). The prevalence of reported atopy was rather high (61%), but only 9% were smokers. Most teachers lived in a suburban area in single family or row houses. Ten per cent of the participants reported discomfort and 6% health effects due to indoor air problems at home. With respect to the workplace, the proportions were higher: 44% for discomfort and 35% for health problems. In the home, 17% of the participants had moisture damage and 9% had smelled mould during the previous year. Only 1% had detected mould growth at home during the previous year. Most participants commuted to work in winter in their own cars (67%) for at least part of the way; using a bus was less common (9%).
Quality assurance
The field blank filters showed a systematic increase in the particle mass concentrations and concentrations of total bacteria during the field measurements of all microenvironments (Table 2). In addition, increases in total fungi, viable fungi and viable bacteria were detected in some field blank filters in the home and work measurements, but not in personal exposure measurements. The increase in absorption coefficient was low in home field blank filters, and the average absorption coefficients in personal and work filters were lower than the absorption coefficient of the clean filter used to adjust the reflectometer.| Field blanks | Weight/µg m−3 | Absorption coefficient × 10−6/m−1 | Total fungi/spores m−3 | Viable fungia/cfu m−3 | Total bacteria/cells m−3 | Viable bacteria/cfu m−3 |
|---|---|---|---|---|---|---|
| a cfu, colony-forming unit. b ni, number of field blanks contaminated by fungi or bacteria. | ||||||
| Homeb (n/ni) | (13) | (13) | (13/1) | (13/0) | (8/8) | (13/4) |
| Average | 2.77 | 20.9 | 312 | 0 | 19749 | 17 |
| Min | −0.74 | −3.56 | 0 | 0 | 4051 | 0 |
| Max | 20.36 | 2.90 | 4051 | 0 | 60765 | 95 |
| SD | 5.63 | 1.79 | 1124 | 0 | 19299 | 32 |
| Personal (n/ni) | (6) | (7) | (4/0) | (7/0) | (4/2) | (7/0) |
| Average | 0.95 | −63.3 | 0 | 0 | 2026 | 0 |
| Min | −0.11 | −1.53 | 0 | 0 | 0 | 0 |
| Max | 1.96 | 1.22 | 0 | 0 | 4051 | 0 |
| SD | 0.97 | 91.9 | 0 | 0 | 2339 | 0 |
| Work (n/ni) | (11) | (12) | (12/3) | (12/1) | (10/9) | (12/7) |
| Average | 4.41 | −12.9 | 3038 | 1 | 20255 | 15 |
| Min | −2.10 | −2.84 | 0 | 0 | 0 | 0 |
| Max | 19.91 | 1.75 | 12152 | 12 | 60765 | 95 |
| SD | 6.73 | 1.29 | 5496 | 3 | 21093 | 26 |
Limits of detection for total fungal and bacterial concentrations were 4051 spores m−3 (for bacteria, cells m−3) in home and personal samples, and 12 152 spores m−3 in workplace samples. For viable fungal and bacterial concentrations, detection limits were 4 cfu m−3 in personal and home samples, and 12 cfu m−3 in workplace samples (cfu, colony-forming unit). The limit of detection in workplace concentrations was higher than in the home or personal samples due to the shorter sampling time (8 h versus 24 h). Calculated detection limits for mass concentrations were 16.9 µg m−3 in home samples, 2.9 µg m−3 in personal samples and 20.2 µg m−3 in workplace samples.
The average values of the relative standard deviation (RSD, %), difference and standard deviation (SD) of parallel duplicates are presented in Table 3. Comparison between duplicates shows that differences were not statistically significant (P = 0.158–1). There was, however, high variation (RSD = 37.1–78.7%) in total fungal and total bacterial duplicates. The variation in the mass concentration was higher in workplace duplicates (RSD = 37.7%) than in home or personal exposure duplicates, but the variation in the absorption coefficient was lowest in workplace duplicates (RSD = 9.9%). The variation in viable fungi was high (RSD = 59.4–75.1%) in all environments, except in workplace samples cultivated on MEA medium (RSD = 15%). Similarly, the variation in viable bacteria was lower in workplace duplicates (RSD = 25.7%) than in home and personal exposure duplicates (RSD = 44.5–46.4%).
| n a | RSDb (%) | Median (RSD, %) | Differencec | SDd | P e | |
|---|---|---|---|---|---|---|
| a n, number of duplicate pairs. b RSD, relative standard deviation, average of duplicate pairs. c Difference, average of duplicate pairs. d SD, standard deviation, average of duplicate pairs. e P, statistical difference between the duplicate measurement results as tested by Wilcoxon signed rank test. f MEA/DG18 (2% malt extract agar/dichloran glycerol 18 agar). | ||||||
| Particle mass | ||||||
| Home | 12 | 28.1 | 13.6 | 5 | 4 | 0.583 |
| Personal | 8 | 12.9 | 6.8 | 3 | 2 | 0.779 |
| Work | 14 | 37.7 | 22.9 | 21 | 15 | 0.646 |
| Absorption coefficient | ||||||
| Home | 12 | 28.0 | 10.2 | 2.4 × 10−6 | 1.7 × 10−6 | 0.158 |
| Personal | 7 | 10.2 | 7.4 | 2.1 × 10−6 | 1.5 × 10−6 | 0.612 |
| Work | 14 | 9.9 | 14.3 | 3.9 × 10−6 | 2.8 × 10−6 | 0.47 |
| Total fungi | ||||||
| Home | 12 | 43.2 | 46.4 | 7489 | 5295 | 0.286 |
| Personal | 4 | 48.1 | 51.3 | 2918 | 2063 | 1 |
| Work | 14 | 37.1 | 46.4 | 9791 | 6923 | 0.433 |
| Total bacteria | ||||||
| Home | 9 | 78.7 | 105.3 | 84482 | 59737 | 0.859 |
| Personal | 4 | 46.1 | 47.6 | 13091 | 9257 | 0.893 |
| Work | 7 | 48.8 | 46.9 | 131904 | 93271 | 1 |
| Viable fungi, MEA/DG18 f | ||||||
| Home | 12 | 75.1/59.4 | 92.9/47.1 | 25.9/21.1 | 18.3/14.9 | 0.858/0.735 |
| Personal | 6 | 68.3/62.5 | 74.9/80.9 | 19.2/18.2 | 13.5/12.8 | 0.344/0.686 |
| Work | 12 | 15.0/51.3 | 0/58.2 | 7.3/17.8 | 5.1/12.6 | 0.285/0.799 |
| Viable bacteria | ||||||
| Home | 12 | 44.5 | 34.3 | 116 | 82 | 0.722 |
| Personal | 6 | 46.4 | 54.9 | 85 | 60 | 0.893 |
| Work | 12 | 25.7 | 23.9 | 443 | 313 | 0.48 |
In parallel tests, the average mass concentrations in outdoor samples were 33 µg m−3 for PEM and 37 µg m−3 for MEM; in indoor samples, the respective values were 11 µg m−3 and 8.5 µg m−3. The average absorption coefficient of the filters in side by side outdoor tests was 1.1 × 10−5 m−1 for both PEM and MEM samples. The difference in the mass concentrations between PEM and MEM monitors was significant (P < 0.001) in the indoor environment, but not significant (P = 0.09) in the outdoor environment. However, the variation in the mass concentration was lower in indoor air samples (RSD = 9.2% for PEM and 5.7% for MEM) than in outdoor samples (RSD = 19% for PEM and 22% for MEM). The difference between the absorption coefficients of the filters in indoor samples was not significant (P = 0.315) and the variations were lower (RSD = 9.9% for PEM and 9.3% for MEM) than the variations in the mass concentrations.
Particle and bioaerosol concentrations
The personal particle mass concentrations (mean, 57 µg m−3) were significantly higher (P = 0.05) than the mass concentrations measured at home (mean, 15 µg m−3) or in the workplace (mean, 30 µg m−3) (Fig. 1). The particle mass concentrations at home were significantly lower (P = 0.05) than the workplace particle mass concentrations. Furthermore, the variation in the mass concentrations at home was lower than that detected in work or personal exposures.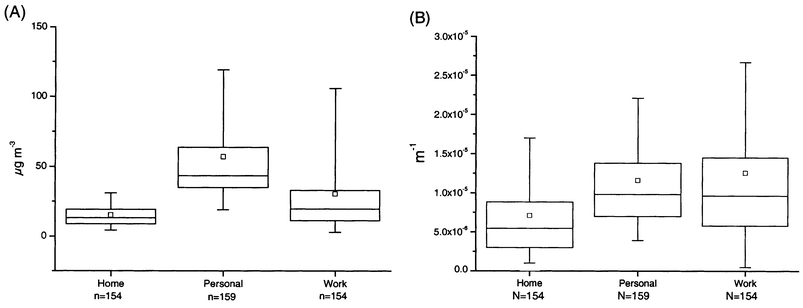 | ||
| Fig. 1 Percentiles and mean values of particle mass concentration (A) and absorption coefficient (B). The boxplot shows the following: □, arithmetic mean; horizontal lines from the bottom, 5%, 25%, 50%, 75% and 95% percentiles. | ||
The absorption coefficients of personal (mean, 1.16 × 10−5 m−1) and workplace (mean, 1.26 × 10−5 m−1) filters showed no significant difference (P = 0.733) (Fig. 1). However, both were significantly higher (P < 0.005) than the values measured in the home filters. Also, the variation in the absorption coefficients of the workplace filters were higher than the variations of the home and personal filters.
The total concentrations of fungi in the work environment [geometric mean (GM) = 9000 spores m−3) were significantly higher (P = 0.05) than those measured at home (GM = 4700 spores m−3) or with personal exposure monitoring (GM = 5700 spores m−3) (Fig. 2), whereas the personal exposures were at the same level as the home concentrations (P > 0.1). The total concentrations of fungi, however, were so low in the home and work environments that the median concentrations were at the detection limit (home, 4051 spores m−3; work, 12 152 spores m−3) of this method.
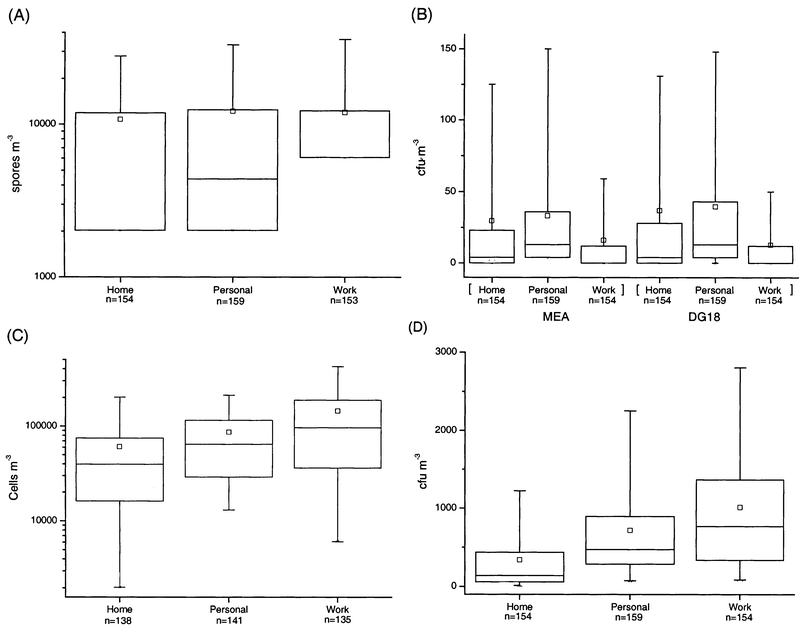 | ||
| Fig. 2 Percentiles and mean concentrations of total fungi (A) and total bacteria (C) and viable fungi (B) and viable bacteria (D). Marks on the plots are the same as in Fig. 1. | ||
The total bacterial concentrations were highest in the work environment (GM = 76 000 cells m−3) and lowest at home (GM = 31 000 cells m−3) (Fig. 2). However, the concentration differences were not significant (P > 0.1). The variation in the total bacterial concentration for personal exposure was lower than that at home or work.
The concentrations of viable fungi in the home environment were significantly lower (GM = 5–6 cfu m−3) (P = 0.05) than the personal exposure (GM = 12 cfu m−3) and significantly higher (P = 0.05) than those found in the work environment (GM = 2–3 cfu m−3) (Fig. 2). The personal exposure concentrations of fungi on DG18 medium were significantly higher (P = 0.05) than the concentrations at work, while the difference was not significant (P > 0.1) for fungi grown on MEA medium. The variation in the concentration of viable fungi on both media (MEA and DG18) for work environment samples was much lower than that for home environment or personal samples.
The viable bacterial concentrations were significantly lower (P = 0.05) in the home environment (GM = 139 cfu m−3) than those measured at work (GM = 413 cfu m−3) or with personal exposure monitoring (GM = 581 cfu m−3) (Fig. 2). There was no significant difference between personal exposure and work concentrations. The variation in the viable bacteria in the home environment was also lower than that for the work environment or personal exposure.
Discussion
This study was designed to provide a novel approach for the documentation of bioaerosol exposure, which has previously been dominated by short time sampling of individual microenvironments. The main emphasis was on total exposures to bioaerosol measured by personal exposure samplers. As far as we are aware, no such total estimations of bioaerosol exposure have previously been reported. Special attention was paid to the quality assurance of the measurements.The study population consisted of a random sample of teachers living in two adjacent townships. The age and gender distribution of the sample was representative of the teaching profession in Finland, where the proportion of female teachers is 70% and the average age is approximately 43 years.
Quality assurance
Field blanks (6% of the total number of samples) were used to evaluate the possible contamination of samples during the field phase. The results showed systematic increases in the particle mass and total bacterial concentration. Slight increases in total fungi, viable fungi and bacteria were also detected in some field blank filters in the home and work measurements. These increases in the blanks may be attributable to contamination during sample handling or the passive sedimentation of particles during the measurement period. The question of undesired sample contamination has not been extensively addressed in the bioaerosol measurement literature,26 but these findings highlight the critical role of this quality assurance aspect of field sampling. Changes in the light absorption coefficient in the field blank filters were so low that this may be due to variation in the quality of the filters.The higher contamination of field blanks in the workplace than at home, excluding bacterial concentrations, may reflect the higher population density in the workplace, especially in classrooms. In both microenvironments, the concentrations in blanks were higher than in personal samples, which is similar to the findings of a study focusing on exposure to PM2.5.38
In samples from work environments, the detection limits were higher due to the shorter sampling time and, consequently, the smaller sample volume than that obtained in the home or personal samples (2 m3versus 6 m3). In addition, different detection limits for mass concentrations in home, personal and work samples were achieved due to field blank contamination, which was used in the calculations of the detection limit.
Duplicate samples (7% of the total number of samples) were collected to investigate the repeatability of the methods. There were no significant differences in the results between the duplicate samples. However, the variations in total fungal and total bacterial duplicates were high. This is in line with earlier observations from field intersample tests and testing of two different samplers, where a rather high variation in total fungal concentrations was also noted.36 However, the variations in fungal and mass concentrations observed in the previous intersample test36 were lower (RSD = 37% for fungi and 10% for particle mass) than in the field duplicates in this study (RSD = 37–48% for fungi and 13–38% for particle mass). This observation emphasises the importance of replicate sampling routines in bioaerosol measurements, although it has not been a commonly applied practice in this type of sampling.26 The variation in the mass concentration in workplace duplicates was higher (RSD = 38%) than that in home duplicates (RSD = 28%) or personal exposure duplicates (RSD = 13%). This could be due to the smaller masses sampled and the greater interference of human activities in values from the workplace versus those in the home. A lower variation of the absorption coefficient in indoor air duplicates (median RSD = 2.9%) has been reported by Janssen et al.25 than that obtained in the home and workplace samples of this study (median RSD = 10–14%), while the variation in personal duplicates was higher (median RSD = 11%) than that in this study (median RSD = 7%).
Particle and bioaerosol concentrations
The personal particle mass exposures were significantly higher than the home and workplace mass concentrations, as shown in previous studies.43,44 Resuspension of particles caused by personal activities (so-called personal cloud) mainly affects coarse particle concentrations.25 The significantly higher particle mass concentrations in the workplace compared to those at home may be caused by human activities, e.g. movement of students in the school environment between classrooms.45 Also, the variation in the mass concentration at work and in personal samples was higher than that at home, which may again be attributable to the movement of occupants around the school building. The frequency distribution of the particle mass concentration in the schools and homes of this study was skewed towards lower concentrations, although the range was similar to that in the button sampler field test study conducted in homes in Cincinnati, OH, USA, where particle mass concentrations varied between 40 and 300 µg m−3.36 PM10 concentrations in indoor air studies in the USA and The Netherlands were higher than those in our study,43,44 but PM2.5 mass concentrations were lower in a study carried out in Helsinki and Amsterdam.25 It is not possible to make a direct comparison between button sampler results and PM10 or PM2.5 results because the sampler inlets have been designed for different efficiencies.The light absorption coefficients of home filters were lower (0.7 × 10−5 m−1) than those of personal and workplace filters (1.3 × 10−5 m−1). The absorption coefficient mainly reflects the amount of soot particles, i.e. emission products from combustion processes such as car engines. Some of the soot particles are so small that they are lost in filter collection. Most of the schools (the workplaces in this study) were located adjacent to traffic routes, which may explain the higher absorption coefficients of work and personal exposure filters than home filters. The absorption coefficients of workplace filters were in line with those reported by Janssen et al.25 The absorption coefficients of the personal exposure filters in our study subjects, working and living in a small rural town, were only slightly lower than those reported from Helsinki and Amsterdam [(1.2–1.3) × 10−5 m−1versus (1.5–1.7) × 10−5 m−1].25 This may be due to the different filter materials and size distribution of the collected particles.
The total concentrations of fungi in the workplace were significantly higher than those sampled in the home environment or by personal exposure monitoring, the last two being at the same level. On the other hand, the concentrations were below or close to the detection limit, which was much higher in workplace samples than in home and personal samples, because of the smaller sampling volume. This may cause a bias, emphasising the concentrations of the work environment at the expense of the lower counts detected in personal and home samples. Total concentrations of fungi measured by the filter method have mainly been reported from work environments, such as farms or during repair work of mouldy buildings, where concentrations of fungi are markedly higher (105–107 spores m−3) than in normal indoor air.46,47 In this study, concentrations of fungi were much lower at home, in schools and in personal samples, being generally around the level of 103–104 spores m−3. Similar concentrations have been found in schools and offices using a filter method, although the range of concentrations was wider: 103–105 spores m−3.48 The average personal exposure (GM) to total fungal particles was about 4000 spores m−3 (range, 0–330 000 spores m−3), while that of viable fungi was 12 cfu m−3 (range, 0–355 cfu m−3); hence, the concentrations of total fungi were approximately 100-fold those of viable fungi. Although the viable fungi were determined from filter samples, where a certain amount of loss is expected due to the desiccation of spores, this ratio of total/viable spores is similar to that assessed for environmental microbes in general.
The concentrations of viable fungi in personal samples were higher than those in the home or at work. This is a new finding, showing that short term stationary measurements probably underestimate the actual personal exposure. This may partly explain the poor correlation between measured fungal levels and health outcomes.28 Home concentrations were significantly higher than those in the work environment, which may be due to the greater prevalence of indoor sources of fungi in homes, such as food handling and fungal microcolonies in bathrooms on temporarily wet surfaces.12,49 Interestingly, the total concentrations of fungi were highest at work, which suggests that a greater fraction of the airborne fungi in the work environment may be aged, dried or otherwise less prone to culturing. The presence of non-cultivatable fungal material may be a result of resuspension due to the movement and activity of building occupants, which was also suggested from the results of the particle mass concentrations. A higher personal exposure to fungi compared to work concentrations was detected on DG18 medium, while the difference was not significant for fungi grown on MEA medium. This may be due to the desiccating effect of filter sampling.50 DG18 medium has a lower water activity than MEA medium, which means that fungi on DG18 favour slightly drier conditions for their growth than those on MEA. The variation in the concentration of viable fungi on both media for the work environment was much lower than that for the home environment or personal exposure. This may indicate the existence of short lasting peaks in fungal concentrations, although their presence cannot be verified with this study design.
The filter method has usually been used to measure viable fungi in work environments in which high concentrations rapidly overload impactor samplers,51 such as farms or during repair work of mouldy structures, where concentrations of viable fungi may be as high as 103–106 cfu m−3.46,47 In this study, the concentrations of viable fungi were low at home, in schools and in personal exposure samples (101 cfu m−3). Slightly higher concentrations of viable fungi have been reported in normal Finnish homes and schools (GM = 18–58 cfu m−3), measured by a six-stage impactor with short term stationary sampling11,14,32 than those measured in this study (GM = 3–12 cfu m−3). The lower viable concentrations in this study are probably explained by the desiccation effect of filter collection.50
The total bacterial concentrations were highest in the work environment and lowest at home, but the differences were not significant. Humans are important sources of indoor air bacteria26,52 and schools are often intensely crowded. This probably explains why the highest bacterial concentrations were found in the workplace. For bacteria, the average personal exposure to total bacterial particles was about GM = 57 000 cells m−3 (range, 0–650 000 cells m−3), and to viable bacterial particles was GM = 468 cfu m−3 (range, 0–3800 cfu m−3). The concentration ratio of viable bacteria counts to total counts of bacteria was close to 1 : 100, which is also commonly found for environmental bacteria in water and soil.53,54 The result also shows that methods based on the counting of viable bacteria badly underestimate the total concentration. It should be noted that even dead or non-cultivatable bacterial aerosols may be important exposure agents. Although infections are strictly associated with live bacteria, non-specific markers of inflammatory responses, and therefore possibly non-infectious health effects, have been shown to be caused by both viable and non-viable bacteria.24
Total bacterial concentrations have been measured by the filter method in work environments, such as woodchip handling, straw handling, grain elevators, pig houses and sawmills. The concentrations of bacteria in such environments are usually high (105–109 cells m−3),55 compared to the findings at home, in schools and in the personal exposure samples (104–105 cells m−3) reported in this study. There are no previous data available on total bacterial concentrations measured with the filter method in home or school environments, but the total bacterial concentrations in this study were generally 102–103-fold greater than previous data obtained on viable bacteria in school and home environments.14,32,56
The lower viable bacterial concentrations in the home environment than in the workplace or personal exposure samples reflects the lower human activity and occupant density in the home than in schools, and is in parallel with the total bacterial concentrations. The concentrations of viable bacteria found in the workplace were comparable to those found in school studies by Liu et al.57 and Meklin et al.,32 and also similar to the concentrations found in normal residences.14,56 However, the concentrations of bacteria found at home were lower than those found in previous studies. This again may be caused by the desiccating effect of filter sampling.50
Conclusions
The particle mass concentration, light absorption coefficient and fungal concentration were higher in personal samples than in stationary samples measured at home or in the workplace. However, bacterial concentrations were highest in heavily populated workplaces. The concentrations of all measured parameters were lowest at home, except for the concentration of viable fungi which was lowest in the workplace. There was extensive variation in the results of fungal and bacterial samples, emphasising the importance of quality assurance (duplicates and field blanks) in microbial field measurements. The use of duplicates and blanks is recommended to control for possible contamination. The microbial results of the filter collection were close to the detection limits, and longer sampling times are needed to achieve better reliability. Despite the high variation in the fungal and bacterial concentrations, personal exposure deserves to be measured to assess the real exposure to bioaerosols. However, stationary samples can provide information on the concentration differences in the microenvironments to which an individual may be exposed.Acknowledgements
This work was funded by The Academy of Finland, Finnish Research Programme on Environmental Health. We are grateful to Ewen MacDonald for reading and commenting on the article.References
- B. Brunekreef, Allergy, 1992, 47, 498 Search PubMed.
- R. E. Dales, I. Schweitzer, S. Bartlett, M. Raizenne and R. Burnett, Indoor Air, 1994, 4, 2 CrossRef.
- I. Pirhonen, A. Nevalainen, T. Husman and J. Pekkanen, Eur. Respir. J., 1996, 9, 2618 CrossRef CAS.
- J. K. Peat, J. Dickerson and J. Li, Allergy, 1998, 53, 120 Search PubMed.
- O. M. Koskinen, T. M. Husman, T. M. Meklin and A. I. Nevalainen, Int. J. Environ. Health Res., 1999, 9, 143 CrossRef.
- A. Hyvärinen, T. Reponen, T. Husman, J. Ruuskanen and A. Nevalainen, Indoor Air, 1993, 3, 337 CrossRef.
- J. A. DeKoster and P. S. Thorne, Am. Ind. Hyg. Assoc. J., 1995, 56, 573 CrossRef.
- J. S. Pastuszka, U. K. T. Paw, D. O. Lis, A. Wlazlo and K. Ulfig, Atmos. Environ., 2000, 34, 3833 CrossRef CAS.
- T. Meklin, T. Reponen, M. Toivola, V. Koponen, T. Husman, A. Hyvärinen and A. Nevalainen, Aerosol Sci. Technol., submitted for publication Search PubMed.
- T. Reponen, A. Hyvärinen, J. Ruuskanen, T. Raunemaa and A. Nevalainen, J. Aerosol Sci., 1994, 25(8), 1595 CrossRef CAS.
- T. Reponen, M. Lehtonen, T. Raunemaa and A. Nevalainen, J. Aerosol Sci., 1992, 23(suppl. 1), 663 CrossRef.
- M. Lehtonen, T. Reponen and A. Nevalainen, Int. Biodeterior. Biodegrad., 1993, 31, 25 CrossRef.
- C. A. Hunter, C. Grant, B. Flannigan and A. F. Bravery, Int. Biodeterior. Biodegrad., 1988, 24, 81.
- A. Hyvärinen, T. Reponen, T. Husman and A. Nevalainen, Cent. Eur. J. Publ. Health, 2001, 9(3), 133 Search PubMed.
- C. G. Bornehag and G. Stridh, in Proceedings of Healthy Buildings, ed. O. Seppänen and J. Säteri, SIY Indoor Air Information, Helsinki, 2000, vol. 1, pp. 437–442 Search PubMed.
- B. B. Jarvis, W. G. Sorenson, E. L. Hintikka, M. Nikulin, Y. H. Zhou, J. Jiang, S. G. Wang, S. Hinkley, R. A. Etzel and D. Deaborn, Appl. Environ. Microbiol., 1998, 64(10), 3620 CAS.
- M. A. Andersson, M. Nikulin, U. Köljalg, M. C. Andersson, F. Rainey, K. Reijula, E.-L. Hintikka and M. Salkinoja-Salonen, Appl. Environ. Microbiol., 1997, 63(2), 387 CAS.
- B. Brunekreef, D. W. Dockery and M. Krzyzanowski, Environ. Health Perspect., 1995, 103(suppl. 2), 3 Search PubMed.
- D. W. Dockery and C. A. Pope, Annu. Rev. Public Health, 1994, 15, 107 CrossRef CAS.
- C. A. Pope, D. W. Dockery and J. Schwartz, Inhal. Toxicol., 1995, 7, 1 CAS.
- P. van Vliet, M. Knape, J. de Hartog, N. Janssen, H. Harssema and B. Brunekreef, Environ. Res., 1997, 74, 122 CrossRef CAS.
- S. Matthias-Maser and R. Jaenicke, J. Aerosol. Sci., 1994, 25(8), 1605 CrossRef CAS.
- A. J. Hälinen, H. Komulainen, R. O. Salonen, M. Ruotsalainen and M. R. Hirvonen, Environ. Toxicol. Pharmacol., 1999, 7, 11 CrossRef CAS.
- M. Hirvonen, M. Ruotsalainen, K. Savolainen and A. Nevalainen, Toxicology, 1997, 124, 105 CrossRef CAS.
- N. A. H. Janssen, J. J. de Hartog, G. Hoek and B. Brunekreef, J. Air Waste Manage. Assoc., 2000, 50, 1133 Search PubMed.
- Bioaerosols: Assessment and Control, ed. J. Macher, American Conference of Governmental Industrial Hygienists, Cincinnati, OH, 1999, ch. 5 Search PubMed.
- H. J. Su, P. C. Wu, H. L. Chen, F. C. Lee and L. L. Lin, Environ. Res., 2001, 85, 135 CrossRef CAS.
- A. P. Verhoeff and H. A. Burge, Ann. Allergy Asthma Immunol., 1997, 78, 544 Search PubMed.
- A. Nevalainen, P. Partanen, E. Jääskeläinen, A. Hyvärinen, O. Koskinen, T. Meklin, M. Vahteristo, J. Koivisto and T. Husman, Indoor Air, 1998, 4, 45 CrossRef.
- T. Sigsgaard, H. L. C. Jensen, E. Nichum, S. Gravesen, L. Larsen and M. Ø. Hansen, in Bioaerosols, Fungi and Mycotoxins: Health Effects, Assessment, Prevention and Control, ed. E. Johanning, Eastern New York Occupational and Environmental Health Center, Albany, New York, 1999, pp. 99–105 Search PubMed.
- K. H. Bartlett, S. M. Kennedy, M. Brauer, B. Dill and C. Vannetten, in Bioaerosols, Fungi and Mycotoxins: Health Effects, Assessment, Prevention and Control, ed. E. Johanning, Eastern New York Occupational and Environmental Health Center, Albany, New York, 1999, pp. 240–244 Search PubMed.
- T. Meklin, T. Husman, A. Vepsäläinen, M. Vahteristo, J. Koivisto, J. Halla-aho, A. Hyvärinen, D. Moschandreas and A. Nevalainen, Indoor Air, in the press Search PubMed.
- U. Haverinen, T. Husman, M. Toivola, J. Suonketo, M. Pentti, R. Lindberg, J. Leinonen, A. Hyvärinen, T. Meklin and A. Nevalainen, Environ. Health Perspect., 1999, 107, 509 Search PubMed.
- L. K. Dotterud, L. H. Vorland and S. Falk, Pediatr. Allergy Immunol., 1995, 6, 181 CAS.
- S. Kalatoor, S. A. Grinshpun and K. Willeke, Atmos. Environ., 1995, 29(10), 1105 CrossRef CAS.
- B. C. Hauck, S. A. Grinshpun, A. Reponen, T. Reponen, K. Willeke and R. L. Bornschein, Am. Ind. Hyg. Assoc. J., 1997, 58, 713 CrossRef CAS.
- M. J. Jantunen, O. Hänninen, K. Katsouyanni, H. Knöppel, N. Kuenzli, E. Lebret, M. Maroni, K. Saarela, R. Srám and D. Zmirou, J. Expo. Anal. Environ. Epidemiol., 1998, 8(4), 495 CAS.
- K. J. Koistinen, A. Kousa, V. Tenhola, O. Hänninen and M. J. Jantunen, J. Air Waste Manage. Assoc., 1999, 49, 1212 Search PubMed.
- National Institute for Occupational Safety and Health (NIOSH), Particulates Not Otherwise Regulated, Total: Method 0500, NIOSH, Cincinnati, OH, 1994 Search PubMed.
- International Standards Organisation, ISO 9835: Ambient Air—Determination of a Black Smoke Index, 1-9, ISO, Geneva, 1993 Search PubMed.
- U. Palmgren, G. Ström, G. Blomquist and P. Malmberg, J. Appl. Bacteriol., 1986, 61, 401 Search PubMed.
- J. H. Zar, in Biostatistical Analysis, Prentice-Hall, NJ, 1999, pp. 224–225 Search PubMed.
- L. Wallace, J. Air Waste Manage. Assoc., 1996, 46, 98 Search PubMed.
- N. A. H. Janssen, G. Hoek, B. Brunekreef, H. Harssema, I. Mensink and A. Zuidhof, Am. J. Epidemiol., 1998, 147, 537 CAS.
- M. Luoma and S. A. Batterman, Indoor Air, 2001, 11, 35 CrossRef CAS.
- S. Lappalainen, M. Nikulin, S. Berg, P. Parikka, E. L. Hintikka and A. L. Pasanen, Atmos. Environ., 1996, 30(17), 3059 CrossRef CAS.
- S. Rautiala, T. Reponen, A. Hyvärinen, A. Nevalainen, T. Husman, A. Vehviläinen and P. Kalliokoski, Am. Ind. Hyg. Assoc. J., 1996, 57, 279 CrossRef CAS.
- M. Toivola, M. Reiman, A. Hyvärinen, T. Meklin and A. Nevalainen, in Bioaerosols, Fungi and Mycotoxins: Health Effects, Assessment, Prevention and Control, ed. E. Johanning, Eastern New York Occupational and Environmental Health Center, Albany, New York, 1999, pp. 453–456 Search PubMed.
- A. L. Pasanen, H. Heinonen-Tanski, P. Kalliokoski and M. J. Jantunen, Atmos. Environ., 1992, 26B, 121.
- Z. Wang, T. Reponen, S. A. Grinshpun, R. L. Górny and K. Willeke, J. Aerosol Sci., 2001, 32, 661 CrossRef CAS.
- A. Nevalainen, K. Willeke, F. Liebhaber, J. Pastuszka, H. Burge and E. Henningson, in Aerosol Measurement, Principles, Techniques and Applications, ed. K. Willeke and P. A. Baron, Van Nostrand Reinhold, New York, 1993, pp. 471–492 Search PubMed.
- I. Goh, J. P. Obbard, S. Viswanathan and Y. Huang, Acta Biotechnol., 2000, 20(1), 67 CrossRef.
- U. Szewzyk, R. Szewzyk, W. Manz and K. H. Schleifer, Annu. Rev. Microbiol., 2000, 54, 81 CrossRef CAS.
- R. M. Atlas and R. Bartha, Microbial Ecology, Fundamentals and Applications, Benjamin/Cummings, Redwood City, CA, 3rd edn., 1993, pp. 165–199 Search PubMed.
- W. Eduard, J. Lacey, K. Karlsson, U. Palmgren, G. Ström and G. Blomquist, Am. Ind. Hyg. Assoc. J., 1990, 51(8), 427 CrossRef CAS.
- T. Reponen, A. Nevalainen, M. Jantunen, M. Pellikka and P. Kalliokoski, Indoor Air, 1992, 2, 26 CrossRef.
- L. J. S. Liu, M. Krahmer, A. Fox, C. E. Feigley, A. Featherstone, A. Saraf and L. Larsson, J. Air Waste Manage. Assoc., 2000, 50, 1957 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
