Expanding the genetic code
Lei Wanga and Peter G. Schultz*b
aDepartment of Chemistry, University of California at Berkeley, Berkeley, CA 94720, USA
bDepartment of Chemistry and the Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.. E-mail: schultz@scripps.edu
First published on 17th December 2001
Abstract
The ability to incorporate unnatural amino acids into proteins directly in living cells will provide new tools to study protein and cellular function, and may generate proteins or even organisms with enhanced properties. Due to the limited promiscuity of some synthetases, natural amino acids can be substituted with close analogs at multiple sites using auxotrophic strains. Alternatively, this can be achieved by deactivating the editing function of some synthetases. The addition of new amino acids to the genetic code, however, requires additional components of the protein biosynthetic machinery including a novel tRNA-codon pair, an aminoacyl-tRNA synthetase, and an amino acid. This new set of components functions orthogonally to the counterparts of the common 20 amino acids, i.e., the orthogonal synthetase (and only this synthetase) aminoacylates the orthogonal tRNA (and only this tRNA) with the unnatural amino acid only, and the resulting acylated tRNA inserts the unnatural amino acid only in response to the unique codon. Using this strategy, the genetic code of Escherichia coli has been expanded to incorporate unnatural amino acids with a fidelity rivaling that of natural amino acids. This methodology is being applied to other cell types and unnatural analogs with a variety of functionalities.
Professor Peter G. Schultz did his undergraduate and graduate work at the California Institute of Technology. His thesis work resulted in the first synthetic molecules that sequence-selectively cleave DNA. In 1985, after postdoctoral studies at the Massachusetts Institute of Technology, he joined the faculty of the University of California at Berkeley, where he was Professor of Chemistry, a Principal Investigator at Lawrence Berkeley National Laboratory and an Investigator of the Howard Hughes Medical Institute. His research—which spans the interface of biology, chemistry, and materials science — includes (a) the development of catalytic antibodies; (b) the application of molecular diversity to problems in biomolecular recognition and catalysis, drug discovery, and materials science; (c) the development of methods for incorporating unnatural amino acids selectively into proteins; (d) single-molecule biological imaging; and most recently (e) studies in functional genomics. He was a founding scientist of Affymax Research Institute and is a cofounder of Symyx Technologies, Syrrx, Phenomix and Kalypsys. His awards include the Waterman Award of the National Science Foundation, membership in the National Academy of Sciences, and the 1994 Wolf Prize in Chemistry. He is currently a Professor of Chemistry at the Scripps Research Institute, and the director of the new Genomics Institute of Novartis Research Foundation in San Diego. |
Lei Wang received his B. S. in organic chemistry and M. S. in physical chemistry from Peking University in 1994 and 1997 respectively, where he conducted research on nanoparticle properties using Scanning Probe Microscopy in Center for Intelligent Material Research. Presently he is a graduate student in the Department of Chemistry at the University of California, Berkeley, under the guidance of Professor Peter G. Schultz, and is investigating in vivo methods for the site-specific incorporation of unnatural amino acids into proteins. |
Introduction
Proteins carry out virtually all of the complex processes of life, from photosynthesis to signal transduction and the immune response. To understand and control these intricate activities, we need to better understand the relationship between the structure and function of proteins. Both site-directed and random mutagenesis, in which specific amino acids in a protein can be replaced with any of the other nineteen common amino acids, have become important tools for this purpose. These methodologies have made possible the generation of proteins with enhanced properties including stability, catalytic activity and binding specificity. Nevertheless, changes in proteins are limited to the 20 common amino acids, most of which have simple functional groups. The ability to include unnatural amino acids with various sizes, acidities, nucleophilicities, hydrophobicities, and other properties into proteins would greatly expand our ability to rationally and systematically manipulate the structures of proteins, both to probe protein function and create new proteins with novel properties.Several strategies have been employed to introduce unnatural amino acids into proteins. The first experiments involved the derivatization of amino acids with reactive side-chains such as Lys, Cys and Tyr, for example, the conversion of lysine to Nε-acetyllysine. Chemical synthesis also provides a straightforward method to incorporate unnatural amino acids, but routine solid-phase peptide synthesis is generally limited to small peptides or proteins with less than 100 residues. With the recent development of enzymatic ligation and native chemical ligation of peptide fragments, it is possible now to make larger proteins.1 A general in vitro biosynthetic method, in which a suppressor tRNA chemically acylated with the desired unnatural amino acid is added to an in vitro extract capable of supporting protein biosynthesis, has been used to site-specifically incorporate over 100 unnatural amino acids into a variety of proteins of virtually any size.2 A broad range of functional groups have been introduced into proteins for studies of protein stability, protein folding, enzyme mechanism, and signal transduction. Although these studies demonstrate that the protein biosynthetic machinery tolerates a wide variety of amino acid side chains, the method is technically demanding, and yields of mutant proteins are low.
The ability to incorporate unnatural amino acids directly into proteins in vivo offers the advantages of high yields of mutant proteins, technical ease, and the potential to study the mutant proteins in cells or possibly in living organisms. In vivo approaches can be generally categorized into multisite substitution and site-specific incorporation. This article will give a brief review of different in vivo strategies, and focus on our efforts to develop a general in vivo site-specific mutagenesis method.
Multisite substitution
Over 50 years ago, it was found that many analogs of natural amino acids inhibit the growth of bacteria. Analysis of the proteins produced in the presence of these amino acid analogs revealed that they had been substituted for their natural counterparts to various extents.3 This occurs because the aminoacyl-tRNA synthetase, the enzyme responsible for the attachment of the correct amino acid to its cognate tRNA, cannot rigorously distinguish the analog from the corresponding natural amino acid. For instance, norleucine is charged by methionyl-tRNA synthetase,4 and p-fluorophenylalanine is charged by phenylalanine-tRNA synthetase.5An in vivo method termed selective pressure incorporation6 was later developed to exploit the promiscuity of wild-type synthetases. An auxotrophic strain, in which the relevant metabolic pathway supplying the cell with a particular natural amino acid is switched off, is grown in minimal media containing limited concentrations of the natural amino acid while transcription of the target gene is repressed. At the onset of stationary growth phase, the natural amino acid is depleted and replaced with the unnatural amino acid analog. Induction of expression of the recombinant protein results in the accumulation of a protein containing the unnatural analog. For example, using this strategy, o, m and p-fluorophenylalanines have been incorporated into proteins, and exhibit two characteristic shoulders in the UV spectrum which can be easily identified;7 trifluoromethionine has been used to replace methionine in bacteriophage λ lysozyme to study its interaction with chitooligosaccharide ligands by 19F NMR;8 and trifluoroleucine has been inserted in place of leucine, resulting in increased thermal and chemical stability of a leucine-zipper protein.9 Moreover, selenomethionine and telluromethionine are incorporated into various recombinant proteins to facilitate the solution of phases in X-ray crystallography.10–13 Methionine analogs with alkene or alkyne functionalities have also been inserted efficiently, allowing for additional modification of proteins by chemical means.14–16
The success of this method depends on the recognition of the unnatural amino acid analogs by aminoacyl-tRNA synthetases, which in general require high selectivity to insure the fidelity of protein translation. Therefore, the range of chemical functionality accessible via this route is limited. For instance, although thiaproline can be incorporated quantitatively into proteins, oxaproline and selenaproline cannot.17 One way to expand the scope of this method is to relax the substrate specificity of aminoacyl-tRNA synthetases, which has been achieved in a limited number of cases. For example, it was found that replacement of Ala294 by Gly in E. coli phenylalanyl-tRNA synthetase (PheRS) increases the size of substrate binding pocket, and results in the acylation of tRNAPhe by p-Cl-phenylalanine (p-Cl-Phe).18 An E. coli strain harboring this mutant PheRS allows the incorporation of p-Cl-phenylalanine or p-Br-phenylalanine in place of phenylalanine.19,20 Similarly, a point mutation Phe130Ser near the amino acid binding site of E. coli tyrosyl-tRNA synthetase was shown to allow azatyrosine to be incorporated more efficiently than tyrosine.21
The fidelity of aminoacylation is maintained both at the level of substrate discrimination and proofreading of non-cognate intermediates and products. Therefore, an alternative strategy to incorporate unnatural amino acids into proteins in vivo is to modify synthetases that have proofreading mechanisms. These synthetases cannot discriminate and therefore activate amino acids that are structurally similar to the cognate natural amino acids. This error is corrected at a separate site, which deacylates the mischarged amino acid from the tRNA to maintain the fidelity of protein translation. If the proofreading activity of the synthetase is disabled, structural analogs that are misactivated may escape the editing function and be incorporated. This approach has been demonstrated recently with the valyl-tRNA synthetase (ValRS).22 ValRS can misaminoacylate tRNAVal with Cys, Thr, or aminobutyrate (Abu); these non-cognate amino acids are subsequently hydrolyzed by the editing domain. After random mutagenesis of the E. coli chromosome, a mutant E. coli strain was selected that has a mutation in the editing site of ValRS. This edit-defective ValRS incorrectly charges tRNAVal with Cys. Because Abu sterically resembles Cys (–SH group of Cys is replaced with –CH3 in Abu), the mutant ValRS also incorporates Abu into proteins when this mutant E. coli strain is grown in the presence of Abu. Mass spectrometric analysis shows that about 24% of valine are replaced by Abu at each valine position in the native protein.
In general, in vivo multisite substitution methods are relatively simple to carry out, and can provide large quantities of engineered proteins. Proteins with modifications at multiple sites are useful for crystallographic studies and NMR determination of protein structure. However, a major limitation is that all sites corresponding to a particular natural amino acid throughout the protein are replaced. The extent of incorporation of the natural and unnatural amino acid may also vary—only in rare cases can quantitative substitution be achieved since it is difficult to completely deplete the cognate natural amino acid inside the cell. Another limitation is that these strategies make it difficult to study the mutant protein in living cells, because the multisite incorporation of analogs often results in toxicity. Finally, this method is applicable in general only to close structural analogs of the common amino acids, again because substitutions must be tolerated at all sites in the genome.
Microinjection of aminoacylated tRNAs into cells
The in vivo site-specific incorporation of unnatural amino acids into proteins was first realized by an extension of the in vitro biosynthetic methodology using microinjection techniques.23,24 A Xenopus oocyte was coinjected with two RNA species made in vitro: an mRNA encoding the target protein with a UAG stop codon at the amino acid position of interest and an amber suppressor tRNA aminoacylated with the desired unnatural amino acid. The translational machinery of the oocyte then inserts the unnatural amino acid at the position specified by UAG. This method has allowed in vivo structure-function studies of integral membrane proteins, which are generally not amenable to in vitro expression systems. Examples include the incorporation of a fluorescent amino acid into tachykinin neurokinin-2 receptor to measure distances by fluorescence resonance energy transfer;25 the incorporation of biotinylated amino acids to identify surface-exposed residues in ion channels;26 the use of caged tyrosine analogs to monitor conformational changes in an ion channel in real time;27 and the use of α-hydroxy amino acids to change ion channel backbones for probing their gating mechanisms.28,29One limitation is inevitably inherited from the in vitro biosynthetic method: the suppressor tRNA has to be chemically aminoacylated with the unnatural amino acid in vitro, and the acylated tRNA is consumed as a stoichiometric reagent during translation and cannot be regenerated. This limitation results in poor suppression efficiency and low protein yields, necessitating highly sensitive techniques to assay the mutant protein such as electrophysiological measurements. Moreover, this method is only applicable to cells that can be microinjected.
A general site-specific incorporation methodology
We have undertaken to develop a general approach for the site-specific incorporation of unnatural amino acids directly into proteins in vivo. Importantly, the unnatural amino acid is added to the genetic repertoire, rather than substituting for one of the common 20 amino acids. This method should (i) allow the site-selective insertion of one or more unnatural amino acids at any desired position of any protein, (ii) be applicable to both prokaryotic and eukaryotic cells, (iii) enable in vivo studies of mutant proteins in addition to the generation of large quantities of purified mutant proteins, and (iv) be adaptable to a large variety of amino acid side chains. Once established, the methodology should be generally accessible to most chemistry and biology laboratories.This approach requires the addition of new components to the biosynthetic machinery including a new tRNA-codon pair, an aminoacyl-tRNA synthetase, and an unnatural amino acid (Fig. 1). A new tRNA must be constructed that is not recognized by the endogenous aminoacyl-tRNA synthetases of the host organism, but functions efficiently in translation (an orthogonal tRNA). This tRNA must deliver the novel amino acid in response to a codon that does not encode any of the common 20 amino acids (a unique codon). A new aminoacyl-tRNA synthetase (an orthogonal synthetase) is also required that aminoacylates the orthogonal tRNA, but does not recognize any of the endogenous tRNAs. This synthetase must aminoacylate the tRNA with only the desired unnatural amino acid but none of the common 20 amino acids. Likewise, the unnatural amino acid cannot be a substrate for the endogenous synthetases. Finally, the amino acid, when added to the growth medium, must be efficiently transported into the cytoplasm. It may also be possible to synthesize the unnatural amino acid biosynthetically or by other modifications to the cellular machinery.
 | ||
| Fig. 1 A general approach for the site-specific incorporation of unnatural amino acids into proteins in vivo. The orthogonal aminoacyl-tRNA synthetase acylates the orthogonal tRNA with an unnatural amino acid. The acylated orthogonal tRNA inserts the unnatural amino acid at the position specified by the unique codon, which is introduced into the gene encoding the protein of interest. | ||
Developing unique genetic codons for unnatural amino acids
The 64 genetic codons code for 20 amino acids and 3 stop signals. Because only one stop codon is needed for translational termination, the other two can in principle be used to encode nonproteinogenic amino acids. The amber stop codon, UAG, has been successfully used in our in vitro biosynthetic system and in Xenopus oocytes to direct the incorporation of unnatural amino acids. Among the 3 stop codons, UAG is the least used stop codon in E. coli. Some E. coli strains contain natural suppressor tRNAs, which recognize UAG and insert a natural amino acid. In addition, these amber suppressor tRNAs have been used in conventional protein mutagenesis. Therefore, it should be possible to use UAG for the incorporation of unnatural amino acids in vivo without significant perturbation of the host E. coli. Because the suppression efficiency for the UAG codon depends upon the competition between the amber suppressor tRNA and the release factor 1 (RF1) (which binds to the UAG codon and initiates release of the growing peptide from the ribosome), it should be possible to modulate the suppression efficiency by either increasing the expression level of the suppressor tRNA or using an RF1 deficient strain. Indeed, several amber suppressor orthogonal tRNAs for use in E. coli and eukaryotic cells have been developed in our group and by others (vide infra).It should also be possible to encode unnatural amino acids with rare codons. For example, when the arginine concentration in an in vitro protein synthesis reaction is reduced, the rare arginine codon, AGG, has proven to be efficient for insertion of Ala by a synthetic tRNA acylated with alanine.30 In this case, the synthetic tRNA competes with the naturally occurring tRNAArg, which exists as a minor species in E. coli. A limitation of using either an amber codon or rare codon as the signal is that only one unnatural amino acid can be encoded at a time. Interestingly, some organisms do not use all triplet codons. An unassigned codon AGA in Micrococcus luteus has been utilized for insertion of amino acids in an in vitro transcription–translation extract.31
An alternative approach is to use extended codons based on frameshift suppression. Four base codons have the potential for insertion of multiple unnatural amino acids into the same protein. For example, four-base codons have been used to incorporate unnatural amino acids into proteins using in vitro biosynthetic methods.30,32 CGGG and AGGU were used to simultaneously incorporate 2-naphthylalanine and an NBD derivative of lysine into streptavidin in vitro with two chemically acylated frameshift suppressor tRNAs.33 In an in vivo study, Moore et al. examined the ability of tRNALeu derivatives with NCUA anticodons to suppress UAGN codons (N can be U, A, G, or C), and found that the quadruplet UAGA can be decoded by a tRNALeu with a UCUA anticodon with an efficiency of 13 to 26% with little decoding in the 0 or −1 frame.34
We have used a combinatorial approach to exhaustively identify tRNAs that efficiently suppress four-base codons.35 A reporter library was constructed in which a serine codon in the β-lactamase gene was replaced by four random nucleotides. A tRNA suppressor library was then generated that consists of derivatives of E. coli tRNASer2 with the anticodon loop (7 nt) replaced with eight or nine random nucleotides. When these two libraries are crossed, an appropriate frameshift suppressor tRNA that decodes the four-base sequence as a single codon results in translation of full-length β-lactamase, rendering the cells resistant to ampicillin. Survival at higher concentrations of ampicillin indicates that the corresponding tRNA has higher suppression efficiency for the four-base codon. Using this selection, four quadruplet codons AGGA, CUAG, UAGA, and CCCU and their cognate suppressor tRNAs were identified that decode only the canonical four-base codon with efficiencies close to that of natural triplet codon suppressors. Novel five- and six-base codon suppressors have also been selected using this strategy.36 These extended codons, some of which are newly identified, should be useful for the incorporation of multiple unnatural amino acids in vitro. Efforts to include them for in vivo protein mutagenesis are also underway. Extended codons also have potential problems: in-frame readthrough of the first three bases as a triplet in the extended codon competes with the overall frameshift suppression. As a result, extended codons based on rare codons or nonsense codons may reduce missense readthrough and frameshift suppression at other unwanted sites.
The ultimate solution for generating unique codons is to develop additional unnatural base pairs, i.e., to expand the existing genetic alphabet. One extra base pair would increase the number of triplet codons from 64 to 125. Essential requirements for third base pair candidates include stable and selective base pairing, efficient enzymatic incorporation into DNA with high fidelity by a polymerase, and the efficient continued primer extension after synthesis of the nascent unnatural base pair. For in vivo usage, the unnatural nucleoside must be membrane permeable and be phosphorylated to form the corresponding triphosphate. In addition, the increased genetic information must be stable and not destroyed by cellular enzymes. Previous efforts by Benner and others took advantage of hydrogen bonding patterns that are different from those in canonical Watson–Crick pairs, the most noteworthy example of which is the iso-C:iso-G pair.37–39 These bases in general mispair to some degree with natural bases and cannot be enzymatically replicated. Kool and co-workers demonstrated that hydrophobic packing interactions between bases can replace hydrogen bonding to drive the formation of base pair.39,40 In an effort to develop an unnatural base pair satisfying all the above requirements, Schultz, Romesberg and co-workers have systematically synthesized and studied a series of unnatural hydrophobic bases. To date, the PICS:PICS (Scheme 1) self-pair is found to be more stable than natural base pairs, and can be efficiently incorporated into DNA by Klenow fragment of E. coli DNA polymerase I (KF).41,42 A 3MN:3MN self-pair can be synthesized by KF with efficiency and selectivity sufficient for biological function.43 However, both bases act as a chain terminator for further replication. A mutant DNA polymerase has been recently evolved that can be used to replicate the PICS self pair.36 In addition, a 7AI self pair can be replicated using a combination of KF and pol β polymerase.44 More unnatural base pairs, including those with changes to both the base and ribose subunit, are currently investigated. For instance, a novel metallobase pair, Dipic:Py, has been developed, which forms a stable pair upon binding Cu(II).45
 | ||
| Scheme 1 Structures of unnatural base pairs driven by forces other than hydrogen bonding. | ||
Generation of orthogonal tRNA–aminoacyl-tRNA synthetase pairs
Extended codons and unnatural base codons have not yet reached the stage of immediate usage in vivo. Therefore, we initially focused on developing orthogonal tRNAs that are amber suppressors for use with the UAG codon. However, the strategies described here should be useful to generate orthogonal tRNAs decoding other codons. Because extended codons and unnatural codons are intrinsically orthogonal to natural codons, it may be relatively easier to develop orthogonal tRNAs for them. We chose E. coli as the host organism to develop this mutagenesis methodology at the outset due to the ease of genetic manipulation, high transformation efficiency, and the availability of established selections and screens.One can attempt to generate an orthogonal tRNA–synthetase pair from an existing E. coli tRNA–synthetase pair. Specifically, the tRNA’s affinity toward its cognate synthetase is eliminated by mutating nucleotides at the tRNA-synthetase interface while preserving its orthogonality to other synthetases and its ability to function in translation. Using the cognate wild-type synthetase as the starting template, a mutant synthetase is then evolved that uniquely recognizes the engineered orthogonal tRNA. Based on an analysis of the X-ray crystal structure of E. coli glutaminyl-tRNA synthetase (GlnRS) complexed with tRNAGln2 , three sites (‘knobs’) in tRNAGln2 were identified which make specific contacts with GlnRS.46,47 These sites were mutated in the tRNA, and mutant suppressor tRNAs containing all possible combinations of knobs 1, 2, and 3 were generated and tested individually by in vitro aminoacylation with GlnRS and in vitro suppression of amber mutants of chorismate mutase. A mutant tRNA (O-tRNA) bearing all three-knob mutations was shown to be orthogonal to all endogenous E. coli synthetases and competent in translation. Next, multiple rounds of DNA shuffling together with oligonucleotide-directed mutagenesis were used to generate libraries of mutant GlnRS’s. These mutant enzymes were selected for their ability to acylate the O-tRNA in vivo using E. coli strain BT235. Only if a mutant GlnRS charges the O-tRNA with glutamine can the genomic amber codon in lacZ be suppressed, enabling BT235 cells to grow on lactose minimal media. Several mutant synthetases surviving each round of selection were purified and assayed in vitro. The ratio of wild-type (wt) tRNAGln acylation to O-tRNA acylation by mutant synthetase decreased significantly upon multiple rounds of selection. However, no mutant E. coli GlnRS′s have been evolved that charge the O-tRNA more efficiently than wild-type E. coli tRNAGln2 : the best mutant evolved after seven rounds of DNA shuffling and selection acylates the O-tRNA at only one-ninth the rate of wt tRNAGln. Devising a negative selection against wt tRNA recognition may eventually lead to mutant synthetases with reversed specificities toward the wt- and O-tRNAs. An ideal orthogonal synthetase should not acylate any wt tRNA, since even modest misacylation of a wt tRNA with an unnatural amino acid will likely result in a lethal phenotype. The lack of such a synthetase candidate, together with the finding that mutations within the tRNA interact in complicated, non-additive ways with respect to both aminoacylation and translation,46 prompted us to examine alternative strategies.
A second strategy for generating an orthogonal tRNA–synthetase pair involves importing a tRNA–synthetase pair from another organism into E. coli. The heterologous synthetase candidate should not charge any E. coli tRNA, and the heterologous tRNA candidate should not be acylated by any E. coli synthetase. In addition, the suppressor tRNA derived from the heterologous tRNA should be orthogonal to all E. coli synthetases. Schimmel et al. reported that E. coli GlnRS (EcGlnRS) does not acylate Saccharomyces cerevisiae tRNAGln (EcGlnRS lacks an N-terminal RNA-binding domain possessed by S. cerevisiae GlnRS (ScGlnRS)).48 This finding prompted us to determine whether the S. cerevisiae amber suppressor tRNAGln (SctRNAGlnCUA ) is also not a substrate for EcGlnRS. In vitro aminoacylation assays showed this to be the case; and in vitro suppression studies show that the SctRNAGlnCUA is competent in translation.49 We further showed that ScGlnRS does not acylate any E. coli tRNA, only the SctRNAGlnCUAin vitro. The degree to which ScGlnRS is able to aminoacylate the SctRNAGlnCUA in E. coli was also evaluated using an in vivo complementation assay. An amber nonsense mutation was introduced at a permissive site in the β-lactamase gene. Suppression of the mutation by an amber suppressor tRNA should produce full-length β-lactamase and confer ampicillin resistance to the cell. When only SctRNAGlnCUA is expressed, cells exhibit an IC50 of 20 μg mL−1 ampicillin, indicating virtually no acylation by endogenous E. coli synthetases; when SctRNAGlnCUA is coexpressed with ScGlnRS, cells acquire an IC50 of about 500 μg mL−1 ampicillin, demonstrating that ScGlnRS acylates SctRNAGlnCUA efficiently in E. coli.49 Therefore, the S. cerevisiae tRNAGlnCUA –GlnRS constitutes an orthogonal pair in E. coli.
This strategy was later applied to a tRNAAsp–AspRS system. S. cerevisiae tRNAAsp is known to be orthogonal to E. coli synthetases.50,51 We demonstrated that an amber suppressor tRNA derived from it (SctRNAAspCUA ) is also orthogonal in E. coli using the aforementioned in vivo β-lactamase assay. However, the anticodon of tRNAAsp is a critical recognition element of AspRS,52 and mutation of the anticodon to CUA results in a loss of affinity of the suppressor for AspRS. Fortunately, an E. coli AspRS E93K mutant has been shown to recognize E. coli amber suppressor tRNAAspCUA about an order of magnitude better than wt AspRS.53 It was speculated that introduction of the related mutation in S. cerevisiae AspRS (E188K) might restore its affinity for SctRNAAspCUA . Indeed, the S. cerevisiae AspRS(E188K) mutant does not acylate E. coli tRNAs, but charges SctRNAAspCUA with moderate efficiency as shown by in vitro aminoacylation experiments.54 Hence the SctRNAAspCUA –ScAspRS(E188K) serves as another orthogonal pair in E. coli, albeit with weak activity. To make this pair valuable for later evolution of a synthetase with unnatural amino acid specificity, expression levels of the synthetase and the tRNA were adjusted, and an RF1 deficient strain was employed, resulting in better selection sensitivity.
A similar approach involves the use of a heterologous synthetase as the orthogonal synthetase but a mutant initiator tRNA of the same organism or a related organism as the orthogonal tRNA. RajBhandary and coworkers found that an amber mutant of human initiator tRNAfMet is acylated by E. coli GlnRS and acts as an amber suppressor in yeast cells only when EcGlnRS is coexpressed.55 This pair thus represents an orthogonal pair for use in yeast. Also, an E. coli initiator tRNAfMet amber mutant was found that is inactive toward any E. coli synthetases. A mutant yeast TyrRS was selected that charges this mutant tRNA, resulting in an orthogonal pair in E. coli.55
The development of multiple orthogonal tRNA–synthetase pairs may allow the simultaneous incorporation of multiple unnatural amino acids using different codons. Moreover, different aminoacyl-tRNA synthetase may be better starting points for generating active sites with particular side chain specificities. Therefore we also turned to tRNA–synthetase pairs that deliver hydrophobic amino acids. A hydrophobic synthetase active site may be more amenable to engineering new substrate specificities for hydrophobic unnatural amino acids such as fluorophores and affinity labels. Attention was focused on tRNATyr–TyrRS pair since prokaryotic and eukaryotic tRNATyr–TyrRS have significant differences: the identity elements of prokaryotic tRNATyr include a long variable arm in contrast to the short arm of eukaryotic tRNATyr. In addition, eukaryotic tRNATyr contains a C1∶G72 positive recognition element whereas prokaryotic tRNATyr has no such consensus base pair. In vitro studies have also shown that tRNATyr of S. cerevisiae and H. sapiens cannot be aminoacylated by bacterial synthetases, nor do their TyrRS aminoacylate bacterial tRNA.56,57 In spite of all these promising features for orthogonality, in vivo β-lactamase complementation assays showed that the amber suppressor tRNATyrCUA derived from both S. cerevisiae and H. sapiens are not orthogonal in E. coli.58
The susceptibility of the suppressor tRNA to acylation by E. coli synthetases is due to the change of one single nucleotide in the anticodon (G34 to C34). Therefore, a tRNA–synthetase pair with identity elements outside of the anticodon, in particular, the tRNATyr–TyrRS pair from the archaebacterial Methanococcus jannaschii was chosen. This TyrRS is missing most of the non-conserved domain binding for the anticodon loop of its tRNATyr, but can discriminate tRNA with C1:G72 from that with G1∶C72. Thus, the M. jannaschii TyrRS (MjTyrRS) aminoacylates S. cerevisiae but not E. coli crude tRNA.59 Using the in vivo complementation assay, we showed that cells expressing the M. jannaschii tRNATyrCUA (MjtRNATyrCUA ) alone survive to an IC50 of 55 μg mL−1 ampicillin; cells coexpressing MjtRNATyrCUA with its TyrRS survive to an IC50 of 1220 μg mL−1 ampicillin.58 This result demonstrates that M. jannaschii tRNATyrCUA –TyrRS is a good candidate for an orthogonal pair in E. coli. Although MjtRNATyrCUA is less orthogonal in E. coli than the SctRNAGlnCUA (IC50 20 μg mL−1), the MjTyrRS has higher aminoacylation activity toward its cognate amber suppressor tRNA.
A general approach was then developed to improve the orthogonality of this MjtRNATyrCUA while preserving its affinity toward MjTyrRS.60 This method consists of a combination of negative and positive selections with a mutant suppressor tRNA library in the absence and presence of the cognate synthetase, respectively. In the negative selection, amber nonsense codon(s) are introduced in a toxic gene at a nonessential position. When a member of the suppressor tRNA library is aminoacylated by endogenous E. coli synthetases (i.e., it is not orthogonal to the E. coli synthetases), the amber codon is suppressed and the toxic gene product produced leads to cell death. Only cells harboring orthogonal tRNAs or non-functional tRNAs can survive. All survivors are then subjected to a positive selection in which an amber codon is placed in a drug resistance gene at a nonessential position. tRNAs are then selected for their ability to be aminoacylated by the coexpressed cognate synthetase and to insert an amino acid in response to this amber codon. Cells harboring non-functional tRNAs, or tRNAs that cannot be recognized by the synthetase of interest will be sensitive to antibiotic. Therefore, only tRNAs that: (i) are not substrates for endogenous E. coli synthetases; (ii) can be aminoacylated by the synthetase of interest; and (iii) are functional in translation will survive both selections.
Eleven nucleotides of the MjtRNATyrCUA that do not interact directly with the TyrRS were randomly mutated to generate a suppressor tRNA library. This tRNA library was passed through a negative selection using the ribonuclease barnase (expression of barnase is toxic to cells) and then a positive selection based on suppression of an amber codon in β-lactamase gene. The best mutant tRNA (mutRNATyrCUA ) selected confers cells an IC50 of 12 μg mL−1 ampicillin, a value similar to that of background readthrough of the amber codon, indicating this mutant tRNA is a much poorer substrate for E. coli synthetase than the wt MjtRNATyrCUA . When the mutRNATyrCUA is coexpressed with MjTyrRS, cells survive to an IC50 of 440 μg mL−1 ampicillin, showing the tRNA is still aminoacylated efficiently by the TyrRS. The low background and high activity of this mutRNATyrCUA –MjTyrRS pair make it an excellent candidate orthogonal pair. This in vivo double selection strategy should be generalizable to additional tRNA–synthetase pairs. As long as an orthogonal synthetase has been identified, in principle it is possible to select an orthogonal suppressor tRNA that can be charged by the synthetase.
Engineering a synthetase with unnatural amino acid specificity
At this stage, the orthogonal synthetase acylates the orthogonal tRNA with a natural amino acid. The substrate specificity of the synthetase must be altered so that only the desired unnatural amino acid, but not any common 20 amino acids are charged. Promiscuity in the orthogonal synthetase will result in mutant proteins with a mixture of natural and unnatural amino acids at the target position. For instance, in an attempt to site-specifically incorporate p-F-Phe, a yeast amber suppressor tRNAPheCUA –phenylalanyl-tRNA synthetase pair was used in a p-F-Phe resistant, Phe auxotrophic E. coli strain.61 Because yeast PheRS does not have high substrate specificity for p-F-Phe, the mutagenesis site is translated with 64–75% p-F-Phe and the remainder as Phe and Lys even in the excess of p-F-Phe added to the growth media. Also, at the Phe codon positions, 7% p-F-Phe is found, indicating that the endogenous E. coli PheRS incorporates p-F-Phe in addition to Phe. Besides its translational infidelity, this approach is not generally applicable to other unnatural amino acids.Modification of the substrate specificity of a synthetase is expected to be difficult due to the high intrinsic fidelity of the natural synthetases and the fact that unnatural amino acids are not required for any cellular function. We have pursued a combinatorial approach to this problem, in which a pool of mutant synthetases is generated from the framework of a wild-type synthetase, and then selected based on their specificity for an unnatural amino acid relative to the common twenty. To isolate such a synthetase, the selection method should be: (i) sensitive, as the activity of ‘hits’ from the initial rounds can be low and the population small; (ii) tunable, since it would be desirable to vary the selection stringency at different selection rounds; (iii) general, so that it can be used for different unnatural amino acids. We describe here several approaches developed in our laboratory to tackle this problem.
A general in vivo selection/screen strategy was developed that is based on the combination of a positive selection followed by a negative selection (Fig. 2). In the positive selection, suppression of the amber codon introduced at nonessential position(s) of the positive marker will allow cells to survive under positive selection pressure. In the presence of both natural and unnatural amino acids, survivors thus encode active synthetases charging the orthogonal suppressor tRNA with either a natural or unnatural amino acid. In the negative selection, those synthetases with specificities for natural amino acids charge the orthogonal tRNA, resulting in suppression of an amber codon in the negative marker and cell death. Since no unnatural amino acid is added, synthetases with specificities for the unnatural amino acid will survive. Survivors passing both selection/screen therefore must encode synthetases charging the orthogonal tRNA with an unnatural amino acid. More mutations are introduced by mutagenesis or DNA shuffling into these synthetase genes to generate a second generation synthetase library, which is used for further rounds of selection until a mutant synthetase with desired activity is evolved.
 | ||
| Fig. 2 Schematic illustration of the general selection/screen for aminoacyl-tRNA synthetases with unnatural amino acid specificities. In the positive selection, active synthetases with either natural or unnatural amino acid specificities are identified; in the negative selection, synthetases with natural amino acid specificities are eliminated. Therefore, only synthetases charging the orthogonal tRNA with the unnatural amino acid can survive both selections/screens. | ||
Our general selection scheme involves a positive selection based on suppression of an amber stop codon at nonessential position in the β-lactamase gene, rendering cells ampicillin resistance; and a negative selection using the ribonuclease barnase as the negative marker.49 An alternative is to replace β-lactamase gene with the chloramphenicol acetyltransferase (CAT) gene, so that chloramphenicol can be applied as the positive selection pressure.54 In contrast to β-lactamase which is secreted into the periplasm, CAT localizes in the cytoplasm; moreover, ampicillin is bacteriocidal while chloramphenicol is bacteriostatic. Because barnase is an extremely toxic protein, it was necessary to control the stringency of the negative selection by introducing a different number of amber codons into the barnase gene. A direct replica plate method was also employed:62 after passing the positive selection, cells are grown in the presence of the either ampicillin or chloramphenicol and the absence of the unnatural amino acid. Those cells that do not survive are isolated from a replica plate supplemented with the unnatural amino acid. No transformation into a second negative selection strain is needed and the phenotype is clear-cut. A limitation of this approach is that the number of colonies that can be handled using replica plates is relatively small. Compared to other potential selection markers, a positive selection based on antibiotic resistance offers the ability to tune selection stringency by varying the concentration of the antibiotic, and to compare the suppression efficiency by monitoring the highest antibiotic concentration cells can survive. More importantly, the growth process is also an enrichment procedure. This can lead to quick accumulation of the desired phenotype; yet can be potentially skewed by other factors affecting growth rates, such as reporter gene reversion.
We have also developed a general fluorescence-activated cell sorting (FACS) based screen with green fluorescent protein (GFP) as the reporter. A T7 RNA polymerase containing amber mutations, together with GFP under control of a T7 promoter are used. Only when the amber codons are suppressed can cells produce functional T7 RNA polymerase and express GFP, rendering cells fluorescence. In the positive screen, fluorescent cells are collected which encode active synthetases charging the orthogonal tRNA with either natural or unnatural amino acids. The selected cells are then diluted and grown in the absence of the unnatural amino acid, and then sorted by FACS for cells without fluorescence, i.e., that express synthetases with specificities for unnatural amino acids only. By setting the collection threshold of the fluorescence intensity, the stringency of both positive and negative screen can be conveniently controlled.36
A direct positive selection specific for a particular unnatural amino acid has also been developed which exploits the high affinity of a monoclonal antibody for an unnatural amino acid displayed on a phage surface (Fig. 3).63 A C3 peptide with an amber mutation is fused to the N-terminus of VSCM13 phage coat protein pIII, such that phage production requires suppression of the amber stop codon. Cells harboring a phagemid that expresses an orthogonal suppressor tRNA and a synthetase library are infected with the C3TAG phage. An active synthetase results in suppression of C3TAG and display of its cognate amino acid on the phage surface. The phage pool is then incubated with immobilized monoclonal antibodies directed against the unnatural amino acid to isolate only those phage carrying the synthetase specific for the unnatural amino acid. In a simulated selection, phage displaying Asp were enriched over 300-fold from a pool of phage displaying Asn using antibodies raised against the Asp-containing epitope.
 | ||
| Fig. 3 Phage-based selection for the incorporation of unnatural amino acids into a surface epitope. E. coli carrying the mutant synthetase library are infected by phage with a stop codon in a gene encoding a surface protein. Phage containing an active synthetase display the unnatural amino acid on the phage surface and are selected with immobilized monoclonal antibodies. | ||
Several in vitro screen methods are also currently being developed. In one such method, a library of mutant synthetases is displayed on the phage, and the phage particles are panned against immobilized sulfamoyl analogs of the aminoacyl adenylate intermediate (Scheme 2). In a preliminary test, M. jannaschii TyrRS was fused to the pIII coat protein of M13 phage. This phage was enriched 1000-fold over a control phage displaying an unrelated antibody after panning against the sulfamoyl analog of tyrosyl adenylate.36 Given that only 0.1 to 1% of the starting TyrRS phage population displays the TyrRS protein, the actual enrichment factor can be as high as 105 to 106.
 | ||
| Scheme 2 The immobilized sulfamoyl analog of the aminoacyl adenylate intermediate used to screen phage-displayed synthetases with unnatural amino acid specificity. | ||
Uptake of unnatural amino acids
A prerequisite for in vivo incorporation of unnatural amino acids is that unnatural analogs must be efficiently uptaken into the cell from the growth media. A rapid screen was developed to assess which unnatural amino acids can be uptaken by cells.49 For some toxic unnatural amino acids, the presence of excess of natural amino acids rescues the ability of cells to grow in the presence of the toxin. These toxic unnatural amino acids are assigned as ‘lethal alleles’. Complementation of the toxic allele, evidenced by the restoration of cell growth, suggests that the nontoxic unnatural amino acids are either uptaken by the cell or competitively inhibit transport of the toxic amino acid. Using this screen, it was found 13 out of 22 analogs of Glu and Gln are likely taken up by the cell, indicating that the E. coli Glu and Gln transport pathways may tolerate significant perturbations in amino acid structure. Recent results also show that a variety of tyrosine analogs can be efficiently uptaken by E. coli.Some specific unnatural analogs with interesting properties may not be uptaken by the cell. Thus it may be necessary to develop methods for transport of unnatural amino acids. One approach takes advantage of peptide permeases, which transport dipeptides and tripeptides across the bacterial inner cell membrane. Peptide permeases are not very side-chain specific, and the Kd values for their substrates are comparable to Kd values of amino acid permeases (0.1–10 μM). The desired unnatural amino acid can be conjugated to a natural amino acid, and the resulting dipeptide is fed to a strain of E. coli deficient in the biosynthesis and uptake of the natural amino acid. The survival of this strain in minimal media would be dependent on the uptake of the dipeptide, and efficient cytoplasmic hydrolysis of the dipeptide to supply the natural amino acid, thus releasing the unnatural amino acid. One can also envisage the use of molecular transporters to actively deliver unnatural amino acids into cells. A candidate transporter is the polyguanidine peptoid derivative, which exhibits significantly enhanced cellular uptake compared to the natural subunit Tat.64 Unnatural amino acids can be covalently attached to this substrate via a native peptide bond, and released by protease cleavage after being translocated into cells by the conjugate.
The first ‘unnatural’ organism
Recently, we have successfully expanded the number of genetically encoded amino acids in E. coli.62 A unique tRNA–aminoacyl-tRNA synthetase pair was generated that, when introduced into E. coli, led to the site-specific incorporation of O-methyl-L-tyrosine (O-met-Tyr) into proteins in response to an amber nonsense codon with a fidelity rivaling that of natural amino acids. The mutRNATyrCUA derived from M. jannaschii tRNATyr (see above) was used as the orthogonal suppressor tRNA; a mutant TyrRS that uniquely charges this tRNA with O-met-Tyr only was evolved from a library of M. jannaschii TyrRS mutants. Five residues in the active site of M. jannaschii TyrRS were all initially mutated to alanine to eliminate wild-type TyrRS contamination (Fig. 4). The resulting inactive Ala5 TyrRS was used as a template for PCR random mutagenesis to generate the mutant TyrRS library with the five residues randomized. A positive selection was applied that is based on suppression of an amber stop codon in the chloramphenicol acetyltransferase (CAT) gene in the presence of the unnatural amino acid. Cells surviving on chloramphenicol were then grown in the presence of chloramphenicol and in the absence of the unnatural amino acid. Those cells that did not survive were isolated from a replica plate supplemented with the unnatural amino acid. The mutant TyrRS genes were isolated from these cells, recombined in vitro by DNA shuffling, and transformed back into E. coli for further rounds of selection with increasing concentrations of chloramphenicol. After two rounds of selection and DNA shuffling, a clone was evolved whose survival in chloramphenicol was dependent on the addition of 1 mM O-methyl-L-tyrosine to the growth media. | ||
| Fig. 4 Stereoview of the active site of TyrRS. Residues from B. stearothermophilus TyrRS are shown in the figure. Corresponding residues from M. jannaschii TyrRS are Tyr32 (Tyr34), Glu107 (Asn123), Asp158 (Asp176), Ile159 (Phe177), and Leu162 (Leu180) with residues from B. stearothermophilus TyrRS in parenthesis. Mutated residues are in yellow. | ||
To demonstrate that the observed phenotype is due to the site-specific incorporation of O-methyl-L-tyrosine by the mutRNATyrCUA –mutant TyrRS pair in response to an amber stop codon, an O-met-Tyr mutant of dihydrofolate reductase (DHFR) was generated and characterized. The third codon of the E. coli DHFR gene was mutated to TAG. When the mutant TyrRS was expressed in the presence of tRNATyrCUA and 1 mM O-met-Tyr, full length DHFR was produced. In the absence of either O-met-Tyr, tRNATyrCUA or mutant TyrRS, no DHFR (<0.1% by densitometry) was observed by analysis with silver-stained SDS-PAGE gel and Western blot (Fig. 5). The identity of the amino acid inserted in response to the TAG codon was confirmed to be O-met-Tyr by mass analysis of both the intact protein and tryptic fragments (Fig. 6). No indication of the incorporation of tyrosine or other amino acids at that position was observed. Analysis of the sequence of the mutant TyrRS revealed the following mutations: Tyr32 → Gln, Asp158 → Ala, Glu107 → Thr, and Leu162 → Pro (Fig. 4). Kinetics of adenylate formation of O-met-Tyr and tyrosine with ATP catalyzed by the mutant TyrRS was analyzed in vitro using a pyrophosphate-exchange assay. The value of kcat/Km of the mutant TyrRS for O-methyl-L-tyrosine is about 100 fold higher than that of tyrosine.
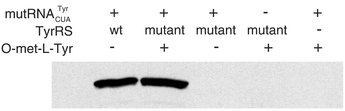 | ||
| Fig. 5 Western blot analysis of the accumulation of E. coli DHFR protein under different conditions. A six-histidine tag was added to the COOH terminus of DHFR, and protein was purified by immobilized metal affinity chromatography. A penta-His antibody was used to detect the six-histidine tag. Expression conditions are notated at the top of each lane. | ||
 | ||
| Fig. 6 Tandem mass spectrum of the NH2-terminal peptide MIY*MIAALAVDR from the mutant DHFR protein. The annotated b (red) or y (blue) ion series confirmed the identity of the residue (Y*) inserted at the TAG codon to be O-methyl-L-tyrosine. The observed value for the monoisotopic mass for the intact mutant protein by FT-ICR MS was 18096.002 daltons, which is within 5 ppm of the theoretical mass of 18095.908 daltons. | ||
Future directions
To demonstrate the generality of this methodology, we are currently evolving more mutant TyrRS with specificities for different tyrosine analogs.36 To date, we have isolated another mutant TyrRS that incorporates L-3-(2-naphthyl)alanine (1) site-specifically (Scheme 3). Promising hits that encode mutant TyrRS’s charging p-amino-L-phenylalanine (2) and p-isopropyl-L-phenylalanine (3) have also been selected. These results suggest that the TyrRS active site is surprisingly amenable to modifications of its specificity. | ||
| Scheme 3 Structures of unnatural amino acids discussed in the text. | ||
In the next stage, a variety of amino acids with novel structural, chemical, and physical properties will be investigated.36 For instance, mutant TyrRS’s with specificities for photoactivatable cross-linkers 4 and 5 have been selected. We are also evolving synthetases with specificities for spin-labeled (6) and fluorescent amino acids (7, 8), amino acids with novel functional groups (9, 10), metal binding amino acids (11), photocaged (12, 13) and photoisomerizable amino acids (14), and biotinylated (15) and glycosylated amino acids (16). To increase the diversity of unnatural amino acids that can be incorporated, a new orthogonal tRNALeu–LeuRS is also being developed.36 The active site of this synthetase should accommodate large amino acid side chains without movement of the polypeptide backbone.
We are also developing orthogonal tRNATyr–TyrRS and tRNATrp–TrpRS pairs for use in mammalian cells.36 Since it is more difficult to generate large libraries in mammalian cells for selection and screening, the active site of mutant TyrRS evolved in E. coli will be transplanted into mammalian cells. Alternatively, a mutant synthetase can be selected using in vitro methods, such as the direct affinity binding approach.
To generate new proteins with novel properties, a ‘random unnatural amino acid mutagenesis’ method is being developed, in which amber stop codons will randomly replace any codon in the gene of a target protein.36 Incorporation of an unnatural amino acid at the TAG codon will result in an unnatural mutant protein library. It is also possible to enable cells to generate unnatural amino acids on their own. We are modifying amino acid biosynthetic pathways or adding new enzymes that convert natural amino acids or metabolic intermediates into unnatural amino acids, thus obviating the addition and uptake of unnatural amino acids.
Conclusion
We have shown it is possible to augment the protein biosynthetic machinery of E. coli to accommodate additional genetically encoded amino acids. Additional orthogonal tRNA–synthetase pairs, as well as new codons, may further expand the number and scope of amino acids that can be incorporated. The ability to introduce novel amino acids into proteins directly in living cells will provide new tools for studies of protein and cellular function and may lead to generation of proteins with enhanced properties.Acknowledgements
We acknowledge support of our work by NIH (GM62159). This is TSRI manuscript No. 14363-CH.Notes and references
- P. E. Dawson and S. B. H. Kent, Annu. Rev. Biochem., 2000, 69, 923 CrossRef CAS.
- V. W. Cornish, D. Mendel and P. G. Schultz, Angew. Chem., Int. Ed. Engl., 1995, 34, 621 CrossRef CAS.
- M. H. Richmond, Bacteriol. Rev., 1962, 26, 398 Search PubMed.
- D. B. Cowie, G. N. Cohen, E. T. Bolton and H. DeRrobinchon-Szulmajst, Biochim. Biophys. Acta, 1959, 34, 39 CAS.
- R. Munier and G. N. Cohen, Biochim. Biophys. Acta, 1959, 31, 378 CAS.
- N. Budisa, C. Minks, S. Alefelder, W. Wenger, F. M. Dong, L. Moroder and R. Huber, FASEB J., 1999, 13, 41 Search PubMed.
- C. Minks, R. Huber, L. Moroder and N. Budisa, Anal. Biochem., 2000, 284, 29 CrossRef CAS.
- H. Duewel, E. Daub, V. Robinson and J. F. Honek, Biochemistry, 1997, 36, 3404 CrossRef CAS.
- Y. Tang, G. Ghirlanda, W. A. Petka, T. Nakajima, W. F. DeGrado and D. A. Tirrell, Angew. Chem., Int. Ed. Engl., 2001, 40, 1494 CrossRef CAS.
- W. A. Hendrickson, J. R. Horton and D. M. Lemaster, EMBO J., 1990, 9, 1665 CAS.
- J. O. Boles, K. Lewinski, M. Kunkle, J. D. Odom, B. Dunlap, L. Lebioda and M. Hatada, Nat. Struct. Biol., 1994, 1, 283 CrossRef CAS.
- N. Budisa, B. Steipe, P. Demange, C. Eckerskorn, J. Kellermann and R. Huber, Eur. J. Biochem., 1995, 230, 788 CAS.
- N. Budisa, W. Karnbrock, S. Steinbacher, A. Humm, L. Prade, T. Neuefeind, L. Moroder and R. Huber, J. Mol. Biol., 1997, 270, 616 CrossRef CAS.
- J. C. M. vanHest and D. A. Tirrell, FEBS Lett., 1998, 428, 68 CrossRef CAS.
- J. C. M. van Hest, K. L. Kiick and D. A. Tirrell, J. Am. Chem. Soc., 2000, 122, 1282 CrossRef CAS.
- K. L. Kiick and D. A. Tirrell, Tetrahedron, 2000, 56, 9487 CrossRef CAS.
- N. Budisa, C. Minks, F. J. Medrano, J. Lutz, R. Huber and L. Moroder, Proc. Natl. Acad. Sci. U.S.A., 1998, 95, 455 CrossRef CAS.
- M. Ibba, P. Kast and H. Hennecke, Biochemistry, 1994, 33, 7107 CrossRef CAS.
- M. Ibba and H. Hennecke, FEBS Lett., 1995, 364, 272 CrossRef CAS.
- N. Sharma, R. Furter, P. Kast and D. A. Tirrell, FEBS Lett., 2000, 467, 37 CrossRef CAS.
- F. Hamano-Takaku, T. Iwama, S. Saito-Yano, K. Takaku, Y. Monden, M. Kitabatake, D. Soll and S. Nishimura, J. Biol. Chem., 2000, 275, 40324 CrossRef CAS.
- V. Doring, H. D. Mootz, L. A. Nangle, T. L. Hendrickson, V. de Crecy-Lagard, P. Schimmel and P. Marliere, Science, 2001, 292, 501 CAS.
- M. W. Nowak, P. C. Kearney, J. R. Sampson, M. E. Saks, C. G. Labarca, S. K. Silverman, W. G. Zhong, J. Thorson, J. N. Abelson, N. Davidson, P. G. Schultz, D. A. Dougherty and H. A. Lester, Science, 1995, 268, 439 CAS.
- D. A. Dougherty, Curr. Opin. Chem. Biol., 2000, 4, 645 CrossRef CAS.
- G. Turcatti, K. Nemeth, M. D. Edgerton, U. Meseth, F. Talabot, M. Peitsch, J. Knowles, H. Vogel and A. Chollet, J. Biol. Chem., 1996, 271, 19991 CrossRef CAS.
- J. P. Gallivan, H. A. Lester and D. A. Dougherty, Chem. Biol., 1997, 4, 739 CrossRef CAS.
- J. C. Miller, S. K. Silverman, P. M. England, D. A. Dougherty and H. A. Lester, Neuron, 1998, 20, 619 CrossRef CAS.
- P. M. England, Y. Zhang, D. A. Dougherty and H. A. Lester, Cell, 1999, 96, 89 CrossRef CAS.
- T. Lu, A. Y. Ting, J. Mainland, L. Y. Jan, P. G. Schultz and J. Yang, Nat. Neurosci., 2001, 4, 239 CrossRef CAS.
- C. H. Ma, W. Kudlicki, O. W. Odom, G. Kramer and B. Hardesty, Biochemistry, 1993, 32, 7939 CrossRef CAS.
- A. K. Kowal and J. S. Oliver, Nucl. Acid. Res., 1997, 25, 4685 Search PubMed.
- T. Hohsaka, D. Kajihara, Y. Ashizuka, H. Murakami and M. Sisido, J. Am. Chem. Soc., 1999, 121, 34 CrossRef CAS.
- T. Hohsaka, Y. Ashizuka, H. Sasaki, H. Murakami and M. Sisido, J. Am. Chem. Soc., 1999, 121, 12194 CrossRef CAS.
- B. Moore, B. C. Persson, C. C. Nelson, R. F. Gesteland and J. F. Atkins, J. Mol. Biol., 2000, 298, 195 CrossRef CAS.
- T. J. Magliery, J. C. Anderson and P. G. Schultz, J. Mol. Biol., 2001, 307, 755 CrossRef CAS.
- P. G. Schultz et al., unpublished results..
- C. Switzer, S. E. Moroney and S. A. Benner, J. Am. Chem. Soc., 1989, 111, 8322 CrossRef CAS.
- J. A. Piccirilli, T. Krauch, S. E. Moroney and S. A. Benner, Nature, 1990, 343, 33 CrossRef CAS.
- E. T. Kool, Curr. Opin. Chem. Biol., 2000, 4, 602 CrossRef CAS.
- K. M. Guckian and E. T. Kool, Angew. Chem., Int. Ed. Engl., 1998, 36, 2825.
- D. L. McMinn, A. K. Ogawa, Y. Q. Wu, J. Q. Liu, P. G. Schultz and F. E. Romesberg, J. Am. Chem. Soc., 1999, 121, 11586 CrossRef CAS.
- A. K. Ogawa, Y. Q. Wu, D. L. McMinn, J. Q. Liu, P. G. Schultz and F. E. Romesberg, J. Am. Chem. Soc., 2000, 122, 3274 CrossRef CAS.
- A. K. Ogawa, Y. Q. Wu, M. Berger, P. G. Schultz and F. E. Romesberg, J. Am. Chem. Soc., 2000, 122, 8803 CrossRef CAS.
- E. J. L. Tae, Y. Q. Wu, G. Xia, P. G. Schultz and F. E. Romesberg, J. Am. Chem. Soc., 2001, 123, 7439 CrossRef CAS.
- E. Meggers, P. L. Holland, W. B. Tolman, F. E. Romesberg and P. G. Schultz, J. Am. Chem. Soc., 2000, 122, 10714 CrossRef CAS.
- D. R. Liu, T. J. Magliery and P. G. Schultz, Chem. Biol., 1997, 4, 685 CrossRef CAS.
- D. R. Liu, T. J. Magliery, M. Pasternak and P. G. Schultz, Proc. Natl. Acad. Sci. U.S.A., 1997, 94, 10092 CrossRef CAS.
- E. F. Whelihan and P. Schimmel, EMBO J., 1997, 16, 2968 CrossRef CAS.
- D. R. Liu and P. G. Schultz, Proc. Natl. Acad. Sci. U.S.A., 1999, 96, 4780 CrossRef CAS.
- B. P. Doctor and J. A. Mudd, J. Biol. Chem., 1963, 238, 3677 CAS.
- Y. Kwok and J. T. Wong, Can. J. Biochem., 1980, 58, 213 CAS.
- R. Giege, C. Florentz, D. Kern, J. Gangloff, G. Eriani and D. Moras, Biochimie, 1996, 78, 605 CrossRef CAS.
- F. Martin, ‘Thesis’, Universite Louis Pasteur, Strasbourg, France, 1995. Search PubMed.
- M. Pastrnak, T. J. Magliery and P. G. Schultz, Helv. Chim. Acta, 2000, 83, 2277 CrossRef CAS.
- A. K. Kowal, C. Kohrer and U. L. RajBhandary, Proc. Natl. Acad. Sci. U.S.A., 2001, 98, 2268 CrossRef CAS.
- K. Wakasugi, C. L. Quinn, N. Tao and P. Schimmel, EMBO J., 1998, 17, 297 CrossRef CAS.
- T. A. Kleeman, D. Wei, K. L. Simpson and E. A. First, J. Biol. Chem., 1997, 272, 14420 CrossRef CAS.
- L. Wang, T. J. Magliery, D. R. Liu and P. G. Schultz, J. Am. Chem. Soc., 2000, 122, 5010 CrossRef CAS.
- B. A. Steer and P. Schimmel, J. Biol. Chem., 1999, 274, 35601 CrossRef CAS.
- L. Wang and P. G. Schultz, Chem. Biol., 2001, 8, 883 CrossRef CAS.
- R. Furter, Protein Sci., 1998, 7, 419 CAS.
- L. Wang, A. Brock, B. Herberich and P. G. Schultz, Science, 2001, 292, 498 CrossRef CAS.
- M. Pastrnak and P. G. Schultz, Bioorg. Med. Chem., 2001, 9, 2373 Search PubMed.
- P. A. Wender, D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman and J. B. Rothbard, Proc. Natl. Acad. Sci. U.S.A., 2000, 97, 13003 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
