Opportunities for ionic liquids in recovery of biofuels
Andrei G. Fadeev†* and Michael M. Meagher
Biological Process Development Facility, The University of Nebraska, Lincoln, NE 68583, USA.. E-mail: mmeagher@unl.edu;; Fax: +1 402-472-1693;; Tel: +1 402-472-2342
First published on 23rd January 2001
Abstract
Room temperature ionic liquids have potential as extractants in recovery of butyl alcohol from fermentation broth; water solubility in ionic liquid and ionic liquid solubility in water are important factors affecting selectivity of butyl alcohol extraction from aqueous solutions.
There has been a renewed interest in processing of biomass into fuels and chemicals via fermentation during the last decade. In that respect, ethanol and acetone–butyl alcohol–ethanol (ABE) fermentations are two of the most attractive bioprocesses. Recovery of alcohols from the fermentation broth is the most energy extensive step in fermentative fuel production. The average energy requirement per litre of EtOH produced is 2446 kcal.1 The amount of energy is about 3 times higher2 for n-BuOH, since ABE fermentation broth contains only about 2 wt% of BuOH vs. 6 wt% of EtOH in yeast fermentation broth. EtOH is currently recovered by distillation. Distillative recovery of BuOH from fermentation broth is not economical. Alternative recovery methods have been considered including: solvent extraction, stripping, pervaporation, etc. None of the methods has been economically proved yet.
We investigated the potential of ionic liquids for extraction of BuOH from aqueous solutions. Room temperature ionic liquids have extremely low vapor pressure and low solubility in water. These properties make them interesting solvents for extraction of organic compounds from aqueous stream.3
Most solvents that have high distribution coefficients for BuOH are flammable, hazardous and toxic to humans and microorganisms.4 Tri-n-butyl phosphate, octan-1-ol and diethyl ether are listed as solvents with high BuOH partition coefficients, 14.6, 7.6, and 4.1, respectively.5 Tri-n-butyl phosphate and octan-1-ol were shown to be toxic to bacteria6 and diethyl ether is extremely flammable.
Solvent selection criteria for liquid–liquid extractions are numerous, including distribution coefficient, density, viscosity, etc. This paper addresses solubility of ionic liquids in water, water coextraction, density and viscosity of extractant. Using the correlation of partitioning data between 1-butyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate ([BMIM][PF6])–water and octan-1-ol–water,3 it could be found that the BuOH partition coefficient in the [BMIM][PF6]–water system should be around 0.8–0.85. Professor K. Seddon advised us that 1-octyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate ([OMIM][PF6]) should have lower solubility in water than [BMIM][PF6].
BMIM[PF6] was prepared according to the procedure described by Huddleston et al.3 The density of the ionic liquid was 1.34 g cm−3.
1-Octyl-3-methyl-1H-imidazol-3-ium chloride was prepared via metathesis reaction of equimolar amounts of 1-methylimidazole and 1-chlorooctane. The two components were mixed in a round-bottom flask equipped with stopcock vacuum adapter, and the air was removed from the flask using a vacuum pump. The reaction was carried out at 80 °C for 3–4 d. The product was washed with ethyl acetate and dried in vacuum. OMIM[PF6] was obtained from 1-octyl-3-methyl-1H-imidazol-3-ium chloride by adding an equimolar amount of hexafluorophosphoric acid. The density of [OMIM][PF6] was 1.21 g cm−3.
To determine distribution coefficients and mutual solubilities, the ionic liquid was mixed with water or BuOH–water solution in an airtight bottle. The mixture was shaken overnight and then left for 1–3 d to achieve phase separation. Mutual solubilities were determined by separating BuOH and water from the IL using a rotary evaporator. The collected BuOH–water solutions were analyzed by GC. BuOH and water distribution coefficients, D, and selectivities, α, were estimated according to eqns. 1 and 2
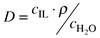 | (1) |
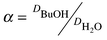 | (2) |
The BuOH distribution coefficient for [BMIM][PF6]–water was found to be very close to the predicted value, 0.85 (Table 1). Distribution coefficients of BuOH for both ionic liquids were similar. Increase in the molecular dimensions of the alkylimidazolium cation decreased mutual solubilities of the ionic liquid and water resulting in higher extractive selectivities for [OMIM][PF6]. Water solubility of [BMIM][PF6] was 6.5 fold higher than that of [OMIM][PF6].
| Aqueous phase, g/1000 g | Ionic liquid phase, g/1000 g | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial aqueous phase composition | BuOH | H2O | [BMIM][PF6] | BuOH | H2O | [BMIM][PF6] | DH2O | DBuOH | α |
| 100% H2O, 23 °C | — | 97.703 | 2.297 | — | 2.116 | 97.884 | 0.029 | — | — |
| 2.01 wt% BuOH, 23 °C | 1.143 | 96.463 | 2.394 | 0.724 | 2.400 | 96.876 | 0.033 | 0.849 | 25.77 |
| 4.74 wt% BuOH, 23 °C | 2.927 | 94.664 | 2.409 | 1.689 | 2.576 | 95.735 | 0.036 | 0.773 | 21.47 |
| 4.97 wt% BuOH, 50 °C | 1.785 | 94.639 | 3.576 | 2.286 | 4.791 | 92.923 | 0.068 | 1.716 | 33.65 |
| Initial aqueous phase composition | BuOH | H2O | [OMIM][PF6] | BuOH | H2O | [OMIM][PF6] | DH2O | DBuOH | α |
| 100% H2O, 23 °C | — | 99.65 | 0.350 | — | 1.520 | 98.480 | 0.018 | — | — |
| 2.01 wt% BuOH, 23 °C | 1.061 | 98.458 | 0.436 | 0.809 | 1.321 | 97.870 | 0.016 | 0.923 | 55.31 |
| 4.74 wt% BuOH, 23 °C | 2.237 | 97.225 | 0.538 | 1.637 | 1.922 | 96.441 | 0.024 | 0.885 | 36.65 |
| 4.97 wt% BuOH, 50 °C | 1.273 | 97.257 | 1.470 | 1.744 | 2.970 | 95.286 | 0.037 | 1.658 | 44.19 |
The larger size of the [OMIM]+ ion also resulted in higher viscosity of [OMIM][PF6] (Fig. 1). The viscosity had strong temperature dependence allowing for fast phase separation at elevated temperature. Higher temperature also improved extractive selectivity, Table 1. BuOH solubility for the ionic liquids under investigation was small, 200–400 mol m−3. It is, however, within the range of solubilities in octanol (200 to 2000 mol m−3). Huddleston et al.3 noted that even low partition coefficients could result in an economical recovery process due to the non-volatility of the extractant.
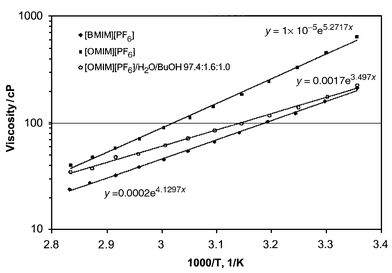 | ||
| Fig. 1 Viscosity vs. 1/T. | ||
Pervaporation is often mentioned as the most promising technology for recovery of organics from aqueous streams, and polydimethylsiloxane membrane is the most extensively studied for organophilic pervaporation.
Pervaporative BuOH recovery from 1 wt% aqueous solution and [OMIM][PF6] was investigated using commercial polydimethylsiloxane membrane MEM-100 (MemPro Co., http://www.MemPro.com). The solution of [OMIM][PF6]–butanol–water 97.4–1.0–1.6 wt% was prepared to simulate the composition of the ionic liquid phase in equilibrium with 1 wt% BuOH–water solution. The pervaporation set-up is described elsewhere.7 Pervaporative selectivity was determined according to the equation:
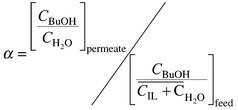 | (3) |
Pervaporation measurements were carried out at 55 °C with permeate pressure 0.01 Torr. Due to the high viscosity of the feed, concentration polarization was anticipated, even though viscosity of [OMIM][PF6]–water–BuOH solution was noticeably lower than that of pure [OMIM][PF6] (Fig. 1). The feed flow through the membrane cell was definitely laminar even at a flow rate of 4 L min−1. Flux through the membrane was 67 g m2 h−1 at selectivity 62. As expected, there was no ionic liquid found in the permeate. Pervaporation of 1 wt% BuOH–water solution at the same conditions showed a flux rate of 110 g m2 h−1 at selectivity 42.
Although the viscosity of [OMIM][PF6]–water–BuOH solution was about 100 fold higher than the viscosity of the BuOH–water mixture, the flux rate through the membrane was only 0.6 fold lower at higher selectivity. BuOH–water ratio in the permeate was close to BuOH–water ratio in ionic liquid feed, suggesting that the membrane did not improve the separation. Distillation is thought to be more economical for BuOH recovery from ionic liquids.
In conclusion, chemical versatility makes ionic liquids interesting solvents for extractive separation in biotechnology. Properties of ionic liquids in that respect remain very much unexplored. We are particularly interested in investigating the mechanism of toxicity of ionic liquids for microorganisms. Preliminary studies of ABE fermentation with [OMIM][PF6] present at saturation level suggest that the IL suppress biological activity in the system.
Acknowledgements
The research was supported by the National Corn Growers Association. The authors are grateful to Dr J. Huang for toxicity tests.Notes and references
- D. Morris and I. Ahmed, How Much Energy Does It Take to Make A Gallon of Ethanol?, Monograph, Institute for Local-Self reliance (ILSR), 1995 (www.ilsr.org).
- M. Matsumura, H. Kataoka, M. Sueki and K. Araki, Energy saving effect of pervaporation using oleyl alcohol liquid membrane, Bioprocess Eng., 1988, 3, 93. Search PubMed.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction, Chem. Commun., 1998, 1765 CAS.
- K. Schungerl, Solvent extraction in biotechnology. Recovery of primary and secondary metabolites, Springer-Verlag, 1994. Search PubMed.
- A. S. Kertes and C. J. King, Extraction chemistry of low molecular weight aliphatic alcohols, Chem. Rev., 1987, 87, 687. Search PubMed.
- S. R. Roffler, H. W. Blanch and C. R. Wilke, In situ recovery of fermentation products, Trends Biotechnol., 1984, 2, 129 CAS.
- A. G. Fadeev, M. M. Meagher, S. S. Kelley and V. V. Volkov, Fouling of poly[1-(trimethylsilyl)prop-1-yne] membranes in pervaporative recovery of butanol from aqueous solutions and ABE fermentation broth, J. Membr. Sci., 2000, 173, 133 CAS.
Footnote |
| † Current address: Santa Fe Science @ Technology, Santa Fe, NM 87505; E-mail: fadeev@sfst.net; Fax: +1 505-474-9489; Phone: +1 505-474-3500. |
| This journal is © The Royal Society of Chemistry 2001 |
