Themed collection Highlighting Canadian research in PCCP

Multiscale modeling of emergent materials: biological and soft matter
Coarse graining techniques enable modelling and simulations of complex biological systems.
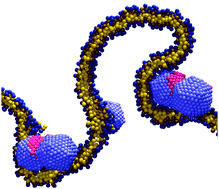
Phys. Chem. Chem. Phys., 2009,11, 1869-1892
https://doi.org/10.1039/B818051B
Cold controlled chemistry
Electron spin orientation in collisions of cold polar molecules can be controlled by superimposed electric and magnetic fields, changing the symmetry of intermolecular interactions.
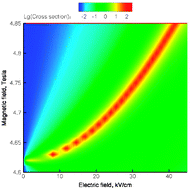
Phys. Chem. Chem. Phys., 2008,10, 4079-4092
https://doi.org/10.1039/B802322K
Fluorescence correlation spectroscopy using quantum dots : advances, challenges and opportunities
Quantum dots and fluorescence correlation spectroscopy: Autocorrelation decays at high excitation rates (blue) show evidence of blinking.
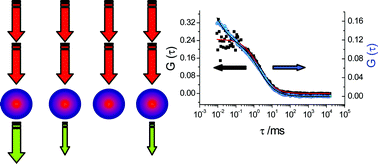
Phys. Chem. Chem. Phys., 2007,9, 1870-1880
https://doi.org/10.1039/B617115J
Crystallographic structure refinement with quadrupolar nuclei: a combined solid-state NMR and GIPAW DFT example using MgBr2
Solid-state NMR and GIPAW DFT calculations reveal the pronounced sensitivity of 79/81Br and 25Mg quadrupolar coupling constants to subtle aspects of solid state structure.
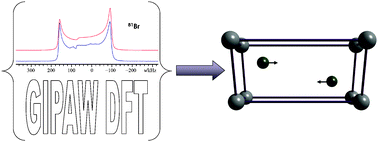
Phys. Chem. Chem. Phys., 2009,11, 7120-7122
https://doi.org/10.1039/B911448N
Evidence for the existence of supercooled ethane droplets under conditions prevalent in Titan’s atmosphere
Evidence for the existence of supercooled liquid ethane aerosols is found in experimental studies that mimic the conditions in Titan’s atmosphere.

Phys. Chem. Chem. Phys., 2008,10, 6211-6214
https://doi.org/10.1039/B811709H
NMR
crystallography of p-tert-butylcalix[4]arene host–guest complexes using 1H complexation-induced chemical shifts
Ultrahigh-field and fast MAS 1H NMR of calixarene complexes show significant complexation-induced chemical shifts that can be used for structure determination.
![Graphical abstract: NMR crystallography of p-tert-butylcalix[4]arene host–guest complexes using 1H complexation-induced chemical shifts](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B805326J&imageInfo.ImageIdentifier.Year=2008)
Phys. Chem. Chem. Phys., 2008,10, 3857-3860
https://doi.org/10.1039/B805326J
Influence of gas-to-particle partitioning on the hygroscopic and droplet activation behaviour of α-pinene secondary organic aerosol
The degree of oxidation of secondary organic aerosol has less effect on droplet activation than previously anticipated.
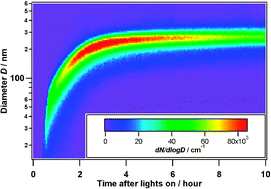
Phys. Chem. Chem. Phys., 2009,11, 8091-8097
https://doi.org/10.1039/B904162A
Static solid-state 14N NMR and computational studies of nitrogen EFG tensors in some crystalline amino acids
The recently introduced direct enhancement of integer spin magnetization (DEISM) method allows static 14N solid-state NMR spectra to be acquired relatively quickly at intermediate field strengths.
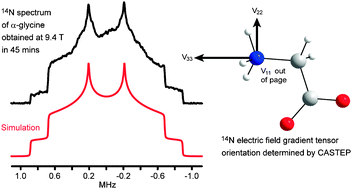
Phys. Chem. Chem. Phys., 2009,11, 7069-7077
https://doi.org/10.1039/B906114B
Molecular dynamics study of the effect of cholesterol on the properties of lipid monolayers at low surface tensions
At low surface tensions an increase in cholesterol concentrations leads to formation of a liquid-condensed phase with low area compressibility.
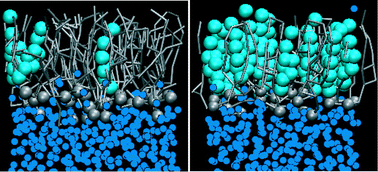
Phys. Chem. Chem. Phys., 2009,11, 1916-1922
https://doi.org/10.1039/B819767A
Probing local structures of siliceous zeolite frameworks by solid-state NMR and first-principles calculations of 29Si–O–29Si scalar couplings
Combined NMR measurements and first-principles calculations of scalar 29Si–O–29Si couplings open new opportunities for framework-structure refinements of zeolites.

Phys. Chem. Chem. Phys., 2009,11, 1825-1837
https://doi.org/10.1039/B815361B
Where does acid hydrolysis take place?
Acid dissociation occurs in the surface zone at an air–water interface via the formation of “solvation clusters” of 3–4 water molecules.
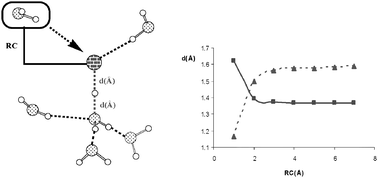
Phys. Chem. Chem. Phys., 2009,11, 857-863
https://doi.org/10.1039/B812070F
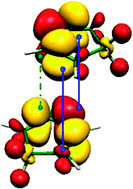
Rationalization of Diels–Alder reactions through the use of the dual reactivity descriptor Δf(r)
An electronic density-based index is used to study both the regioselectivity and the stereoselectivity of Diels–Alder reactions.
Phys. Chem. Chem. Phys., 2008,10, 7239-7246
https://doi.org/10.1039/B810343G
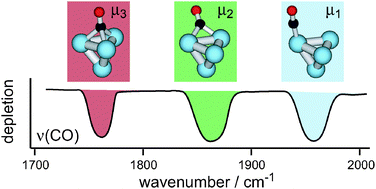
The adsorption of CO on group 10 (Ni, Pd, Pt) transition-metal clusters
Vibrational spectroscopy of CO bound to small metal clusters in the gas phase reveals characteristic differences in the CO binding geometries for Ni, Pd, and Pt.
Phys. Chem. Chem. Phys., 2008,10, 6144-6149
https://doi.org/10.1039/B808341J
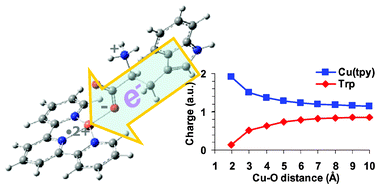
Dissociations of copper(II)-containing complexes of aromatic amino acids : radical cations of tryptophan , tyrosine , and phenylalanine
Charge- and radical-induced fragmentations of Cu(II)-containing complexes of aromatic amino acid are studied by mass spectrometry and density functional theory.
Phys. Chem. Chem. Phys., 2008,10, 5908-5918
https://doi.org/10.1039/B807692H
Aqueous peptides as experimental models for hydration water dynamics near protein surfaces
We use hydrophilic and amphiphilic peptides as models for understanding hydration dynamics near chemically heterogeneous protein surfaces.
Phys. Chem. Chem. Phys., 2008,10, 4903-4908
https://doi.org/10.1039/B806995F
On the lifetimes and physical nature of incompletely relaxed electrons in liquid water
Electron hydration dynamics remains very controversial. We reveal that these controversies are to a great extent due to the universal existence of a coherence spike in pump–probe spectroscopic kinetics traces.
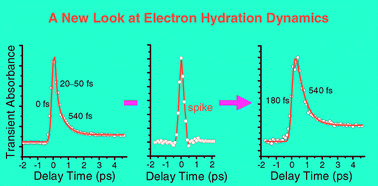
Phys. Chem. Chem. Phys., 2008,10, 4463-4470
https://doi.org/10.1039/B806287K
Analytic three-dimensional ‘MLR’ potential energy surface for CO2–He, and its predicted microwave and infrared spectra
Fits to ab initio interaction energies yield analytic two- and three-dimensional potential-energy surfaces for CO2–He whose predicted microwave and infrared spectra are compared with experiment.
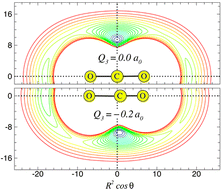
Phys. Chem. Chem. Phys., 2008,10, 4128-4137
https://doi.org/10.1039/B800718G
Computational comparison of the stacking interactions between the aromatic amino acids and the natural or (cationic) methylated nucleobases
The MP2/6-31G*(0.25) stacking interactions were investigated between the aromatic amino acids and ten common methylated nucleobases.
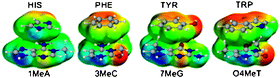
Phys. Chem. Chem. Phys., 2008,10, 2801-2812
https://doi.org/10.1039/B718621E
Adsorption of fibrinogen on a biomedical-grade stainless steel 316LVM surface: a PM-IRRAS study of the adsorption thermodynamics, kinetics and secondary structure changes
PM-IRRAS demonstrated that adsorption of fibrinogen on a biomedical-grade stainless steel surface results in significant changes in secondary structure of the protein.
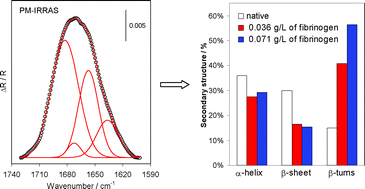
Phys. Chem. Chem. Phys., 2008,10, 2502-2512
https://doi.org/10.1039/B719371H
Measurement and revised analysis of the torsional combination band of the nonpolar N2O dimer at 2249 cm−1
Reanalysis of an N2O dimer torsional combination band gives a band origin which is 1.53 cm−1 higher than previously reported.
Phys. Chem. Chem. Phys., 2008,10, 1658-1661
https://doi.org/10.1039/B718509J
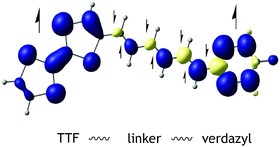
Towards understanding of magnetic interactions within a series of tetrathiafulvalene–π conjugated-verdazyl diradical cation system: a density functional theory study
Exchange coupling for a set of ten tetrathiafulvalene (TTF) and verdazyl diradicals connected by different linkers are investigated using DFT methods.
Phys. Chem. Chem. Phys., 2008,10, 857-864
https://doi.org/10.1039/B715315E
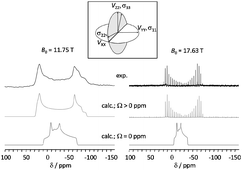
Molybdenum magnetic shielding and quadrupolar tensors for a series of molybdate salts: a solid-state 95Mo NMR study
The Mo shielding and nuclear quadrupolar interactions are characterized for solid molybdate salts using 95Mo NMR at high magnetic fields.
Phys. Chem. Chem. Phys., 2008,10, 574-581
https://doi.org/10.1039/B713276J
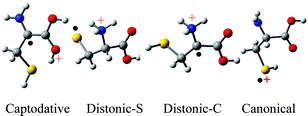
The cysteine radical cation : structures and fragmentation pathways
Relative energies are: Captodative < Distonic-S < Distonic-C (SH2) < Distonic-C < Canonical < Carboxy (COO˙).
Phys. Chem. Chem. Phys., 2008,10, 281-288
https://doi.org/10.1039/B712628J
Chirality transfer through hydrogen-bonding: Experimental and ab initio analyses of vibrational circular dichroism spectra of methyl lactate in water
The observation of chirality transfer from chiral solute to water solvent in VCD spectra opens a new spectral window to probe the solvent effect.
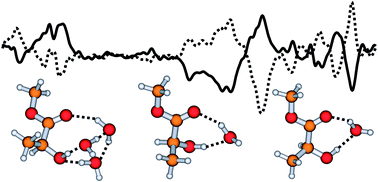
Phys. Chem. Chem. Phys., 2007,9, 3127-3135
https://doi.org/10.1039/B703368K
A computational characterization of the hydrogen-bonding and stacking interactions of hypoxanthine
Hydrogen-bonding and stacking binding strengths of hypoxanthine, a potential universal nucleobase, were compared using a variety of computational methodologies.
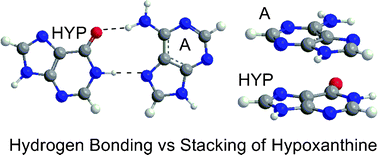
Phys. Chem. Chem. Phys., 2007,9, 497-509
https://doi.org/10.1039/B606388H
About this collection
PCCP is delighted to announce that the Canadian Society for Chemistry (CSC) is now one of its co-owner societies.
Our partnership with CSC now brings the total number of PCCP owner societies to 17, which includes chemical societies from across the globe.
To celebrate this new partnership, we have created a collection of 25 top cited articles from authors based in Canada to showcase some of the great Canadian research published in PCCP. You can read the articles for free now!