Construction of regio- and stereoregular poly(enaminone)s by multicomponent tandem polymerizations of diynes, diaroyl chloride and primary amines†
Abstract
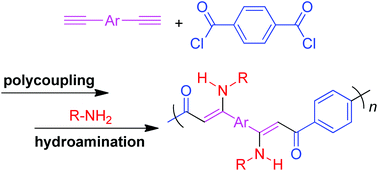
Polyhydroaminations for the synthesis of stable nitrogen-substituted conjugated polymers with well-defined structures remain a great challenge and the control of the regio- and stereochemistry of the enamine product of the hydroamination is non-trivial. Herein we report an efficient tandem polymerization of alkynes, carbonyl chlorides and primary amines to afford regio- and stereoregular conjugated poly(enaminone)s. The atom-economical one-pot sequential polycoupling–hydroamination polymerization catalyzed by Pd(PPh3)2Cl2/CuI proceeded smoothly under mild conditions, furnishing nitrogen-substituted conjugated polymers with high molecular weights (up to 46 100) and high regio-/stereoregularities (100%) in nearly quantitative yields (up to 99%). The single crystal structure of the model compound, together with the NMR spectra comparison of the model compound and polymers provided direct insight into the stereoselectivity of the polymerization, verifying the sole Z-vinylene isomer of the polymers. Through the exquisite structural design strategy of the intramolecular hydrogen bond of the resulting hydroamination product, the tautomerization between enamine and imine as well as E/Z isomerization was successfully avoided, providing products with high chemical stability and sole Z-vinylene isomers. The conjugated polymers display excellent solubility in common organic solvents, good film-forming ability, and high thermal stability. The hydrogen bond formation of the polymer helps to block the potential photo-induced electron transfer process and the polymer shows a unique aggregation-enhanced emission phenomenon: their solutions are weakly emissive, while their nanoaggregates or thin films are brightly emissive. Furthermore, thin films of the polymers enjoy high refractive indices (1.9103–1.6582) in a wide wavelength region of 400–1000 nm, which can be further modulated by UV irradiation. Meanwhile, well-resolved fluorescent photopatterns of the polymers can be fabricated through the UV irradiation of thin films via a copper photomask.

 Please wait while we load your content...
Please wait while we load your content...