Synthesis, structure and N–N bonding character of 1,1-disubstituted indazolium hexafluorophosphate†
Abstract
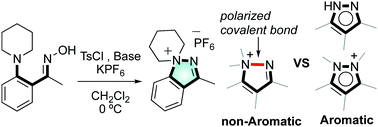
1,1-Disubstituted indazolium hexafluorophosphates were synthesized via intramolecular electrophilic amination reactions under mild conditions. The crystal structures were determined and are consistent with the presence of a stable N–N bond, which can be cleaved by hydrogenation. Both experimental and computational studies suggest a covalent bonding character of the N–N bond, with diminished aromaticity of the newly formed pyrazolium ring due to the quaternary ammonium atom (N1), in contrast to the aromatic character of the parent indazole.


 Please wait while we load your content...
Please wait while we load your content...