The ligand 2,4,6-tris(dimethoxyphosphonate)-1,3,5-triazine L has been synthesized and its single crystal X-ray structure determined. The occurrence of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π intermolecular interactions, suggested by the short P
O⋯π intermolecular interactions, suggested by the short P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯triazine distances of 3.16–3.35 Å, is observed. The electrochemical reduction of the ligand shows its electron acceptor character by the formation of a stable radical anion. The hyperfine structure observed in the EPR spectra, combined with a theoretical DFT study, evidences the full delocalization of the unpaired electron mainly on the triazine core, with some participation of the phosphonate groups. Theoretical calculations are in agreement with the experimental values of the hyperfine coupling constants of 11.81 G for Aiso–31P and 1.85 G for Aiso–14N. Homopolymetallic complexes, formulated as {L[Cu(hfac)2]3} (1), 1∞{L2[Co(hfac)2]3} (2) and 1∞{L2[Mn(hfac)2]3} (3) (hfac = hexafluoroacetylacetonate), have been synthesized and structurally characterized.
O⋯triazine distances of 3.16–3.35 Å, is observed. The electrochemical reduction of the ligand shows its electron acceptor character by the formation of a stable radical anion. The hyperfine structure observed in the EPR spectra, combined with a theoretical DFT study, evidences the full delocalization of the unpaired electron mainly on the triazine core, with some participation of the phosphonate groups. Theoretical calculations are in agreement with the experimental values of the hyperfine coupling constants of 11.81 G for Aiso–31P and 1.85 G for Aiso–14N. Homopolymetallic complexes, formulated as {L[Cu(hfac)2]3} (1), 1∞{L2[Co(hfac)2]3} (2) and 1∞{L2[Mn(hfac)2]3} (3) (hfac = hexafluoroacetylacetonate), have been synthesized and structurally characterized.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π intermolecular interactions, suggested by the short P
O⋯π intermolecular interactions, suggested by the short P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯triazine distances of 3.16–3.35 Å, is observed. The electrochemical
O⋯triazine distances of 3.16–3.35 Å, is observed. The electrochemical 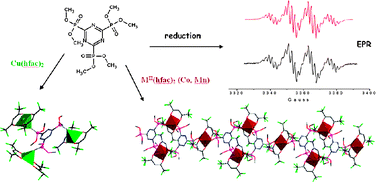

 Please wait while we load your content...
Please wait while we load your content...