The preparation of a series of amidinato and guanidinato zinc halide complexes incorporating ligands of varying steric bulk is described, and their thermal stabilities compared. Salt elimination reactions between [M(Giso)] (M = K or Li; Giso = [(ArN)2CNCy2]−, Ar = 2,6-diisopropylphenyl, Cy = cyclohexyl) and ZnX2 (X = I or Br) have yielded the monomeric complexes [(Giso)ZnI] and [(Giso)Zn(μ-Br)2Li(OEt2)2]. Both have been crystallographically characterised and the former shown to slowly decompose in solution at ambient temperature to give the carbodiimide, ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
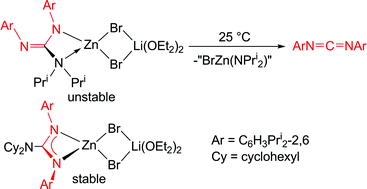
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr. In contrast, reactions between alkali metal complexes of a less bulky guanidinate, [M(Priso)] (Priso = [(ArN)2CNPri2]−) and ZnX2 have yielded [(IZn)2(μ-NPri2){μ-N,N′-(NAr)2CH}] and [(Priso)Zn(μ-Br)2Li(OEt2)2]. The latter decomposes in solution at ambient temperature, generating ArN
NAr. In contrast, reactions between alkali metal complexes of a less bulky guanidinate, [M(Priso)] (Priso = [(ArN)2CNPri2]−) and ZnX2 have yielded [(IZn)2(μ-NPri2){μ-N,N′-(NAr)2CH}] and [(Priso)Zn(μ-Br)2Li(OEt2)2]. The latter decomposes in solution at ambient temperature, generating ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr, which was also produced in the preparation of the former. Analogies are drawn between the decomposition of [(Priso)Zn(μ-Br)2Li(OEt2)2] and the carbonic anhydrase catalysed dehydration of bicarbonate. Two bulky amidinato zinc complexes, [{(Piso)Zn(μ-Br)}2] and [Zn(Piso)2] (Piso = [(ArN)2CBut]−) have been prepared, structurally characterised and shown to be markedly more thermally stable than the zinc guanidinate compounds. Attempts to reduce several of the zinc(II) halide complexes to dimeric zinc(I) compounds were so far unsuccessful, in all cases leading to the deposition of zinc metal.
NAr, which was also produced in the preparation of the former. Analogies are drawn between the decomposition of [(Priso)Zn(μ-Br)2Li(OEt2)2] and the carbonic anhydrase catalysed dehydration of bicarbonate. Two bulky amidinato zinc complexes, [{(Piso)Zn(μ-Br)}2] and [Zn(Piso)2] (Piso = [(ArN)2CBut]−) have been prepared, structurally characterised and shown to be markedly more thermally stable than the zinc guanidinate compounds. Attempts to reduce several of the zinc(II) halide complexes to dimeric zinc(I) compounds were so far unsuccessful, in all cases leading to the deposition of zinc metal.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr. In contrast, reactions between
NAr. In contrast, reactions between ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr, which was also produced in the preparation of the former. Analogies are drawn between the
NAr, which was also produced in the preparation of the former. Analogies are drawn between the 

 Please wait while we load your content...
Please wait while we load your content...