Reorganization energies and pre-exponential factors in the one-electron electrochemical and homogeneous oxidation of phenols coupled with an intramolecular amine-driven proton transfer†
Abstract
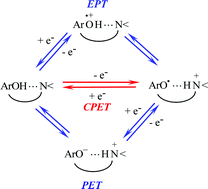
Temperature variations of the kinetics of the electrochemical and homogeneous oxidation of the title compounds give rise to Arrhenius plots, the slopes of which give access to the heavy-atom (including solvent) reorganization energies. Information on the role of proton transfer in the dynamics of the concerted proton–electron transfer reaction (CPET) is potentially contained in the pre-exponential factor. Previous analyses of the problem were based on equalling the pre-exponential factor in the absence of barrier for proton transfer with the collision frequency. Taking into account that the reaction may take place at various distances from the electrode surface increases the value of this limiting pre-exponential factor. Strategies are discussed for evaluating the impact of proton transfer in the CPET kinetics by comparing the experimental pre-exponential factor with pre-exponential factors characterizing simple outersphere electron transfers.
 Please wait while we load your content...
Please wait while we load your content...